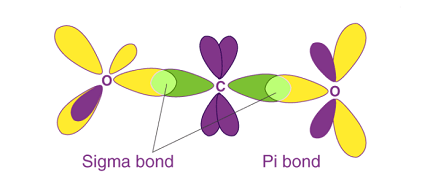
σ and π-bonds are two types of covalent bonds that form between atoms when they share electrons.
A σ-bond is formed when two atoms directly overlap their atomic orbitals along the line connecting their nuclei. This type of bond is characterized by the electron density being concentrated in the region directly between the two nuclei. A σ-bond can form between any two orbitals, whether they are s, p, d, or hybrid orbitals.
A π-bond is formed when two atomic orbitals that are parallel to each other overlap, creating a region of electron density above and below the plane of the atoms. This type of bond is characterized by a cloud of electron density that is concentrated above and below the line connecting the nuclei of the atoms. π-bonds typically form between p orbitals that are oriented in the same direction.
In general, a double bond consists of one σ-bond and one π-bond, while a triple bond consists of one σ-bond and two π-bonds. The presence of π-bonds in a molecule can affect its properties, such as its reactivity and the way it interacts with other molecules.
What is Required Basic Principles of Organic Chemistry σ and π-bonds
The basic principles of organic chemistry that are required to understand σ and π-bonds include:
- Valence shell electron pair repulsion (VSEPR) theory: This theory explains how the shapes of molecules are determined by the arrangement of electron pairs around the central atom. The theory predicts the geometry of molecules based on the number of bonding and nonbonding electron pairs.
- Hybridization: This concept explains how the atomic orbitals of an atom combine to form hybrid orbitals, which can participate in bonding. Hybridization explains the shapes of molecules and the nature of σ-bonds.
- Molecular orbital theory: This theory explains how σ and π-bonds form between atoms. Molecular orbital theory predicts the energy levels and stability of molecular orbitals based on the combination of atomic orbitals.
- Resonance: This concept explains how molecules can have multiple valid Lewis structures due to the delocalization of electrons in π-bonds. Resonance structures are used to represent the actual distribution of electrons in a molecule.
- Electronegativity: This property of atoms determines the polarity of σ and π-bonds in a molecule. Electronegativity values are used to predict the direction of electron density in a molecule, which affects the reactivity and physical properties of the molecule.
Who is Required Basic Principles of Organic Chemistry σ and π-bonds
The principles of organic chemistry, including σ and π-bonds, are required knowledge for anyone studying or working in the field of chemistry, particularly those working in organic chemistry or biochemistry. This includes:
- Students of chemistry and related fields, such as biology and chemical engineering, who are learning about the properties and reactions of organic compounds.
- Chemists and researchers working in the pharmaceutical, biotechnology, and materials science industries, who use organic compounds as the building blocks for drug molecules, biomaterials, and other advanced materials.
- Professors and educators who teach courses in organic chemistry and related topics.
- Scientists in government and academic research institutions who investigate the properties and behavior of organic compounds, including their reactivity, stability, and environmental impact.
Overall, a solid understanding of the principles of organic chemistry and the nature of σ and π-bonds is essential for anyone working in the field of chemistry or related disciplines.
When is Required Basic Principles of Organic Chemistry σ and π-bonds
The principles of organic chemistry, including σ and π-bonds, are required knowledge at various stages of education and in different fields of work.
In academic settings, students typically encounter these concepts in introductory or advanced courses in organic chemistry, which may be part of a chemistry, biochemistry, or related program. These courses may be taken at the undergraduate or graduate level, depending on the student’s academic background and career goals.
In the workplace, knowledge of organic chemistry principles is essential for chemists and researchers working in the pharmaceutical, biotechnology, and materials science industries, as well as in academia and government research institutions. These professionals use the principles of organic chemistry to design, synthesize, and analyze organic compounds and materials for a wide range of applications.
Overall, the principles of organic chemistry and the nature of σ and π-bonds are required knowledge for anyone working with organic compounds, whether in academic research, industry, or other fields.
Where is Required Basic Principles of Organic Chemistry σ and π-bonds
The principles of organic chemistry, including σ and π-bonds, are required knowledge in various educational settings and fields of work.
In educational settings, the principles of organic chemistry are typically taught in chemistry or biochemistry courses at colleges and universities. These courses may be part of undergraduate or graduate programs in chemistry, biochemistry, or related fields.
In the workplace, knowledge of organic chemistry principles is essential for chemists and researchers working in the pharmaceutical, biotechnology, and materials science industries, as well as in academic and government research institutions. These professionals may work in laboratories, research facilities, or manufacturing plants, depending on their field of expertise.
In addition to traditional educational and workplace settings, there are also online resources and courses available for those who want to learn more about organic chemistry principles, including σ and π-bonds. These resources may include online courses, tutorials, textbooks, and research articles.
Overall, the principles of organic chemistry and the nature of σ and π-bonds are required knowledge for anyone working with organic compounds, whether in an academic, industrial, or other setting.
How is Required Basic Principles of Organic Chemistry σ and π-bonds
The principles of organic chemistry, including σ and π-bonds, are typically taught through a combination of lectures, textbooks, laboratory experiments, and online resources.
In a typical organic chemistry course, students will learn the basics of chemical bonding, including the nature of σ and π-bonds, and how they relate to the properties and reactivity of organic compounds. Students will also learn about the structure and behavior of functional groups, which are the reactive parts of organic molecules.
Laboratory experiments are an essential component of organic chemistry education, allowing students to apply the concepts they learn in the classroom to real-world situations. In these experiments, students learn how to synthesize organic compounds, purify them, and analyze their properties using a variety of techniques, such as spectroscopy and chromatography.
Online resources, such as tutorials and interactive simulations, can also be useful for learning about organic chemistry principles, including σ and π-bonds. These resources can be particularly helpful for students who want to review concepts or practice problems outside of the classroom.
Overall, learning the principles of organic chemistry and the nature of σ and π-bonds requires a combination of theoretical and practical knowledge, as well as an understanding of the underlying principles of chemistry and physics.
Case Study on Basic Principles of Organic Chemistry σ and π-bonds
Here is a case study that demonstrates the importance of understanding the basic principles of organic chemistry, including σ and π-bonds:
John is a graduate student studying organic chemistry. He is working on a project to synthesize a new drug molecule, which he hopes will be more effective than existing treatments for a certain disease. To do this, he needs to design a molecule that can interact with specific targets in the body, and that can be synthesized efficiently and cost-effectively.
John begins by using his knowledge of organic chemistry to design a molecule that contains specific functional groups, which he knows will be important for the molecule’s activity. He then uses computer modeling software to optimize the structure of the molecule, taking into account factors such as its stability, reactivity, and solubility.
Once John has designed the molecule, he must synthesize it in the lab. This involves a series of reactions that convert starting materials into the desired product. To do this, John must carefully control the reaction conditions, such as temperature, pressure, and reagent concentrations, to ensure that the reactions proceed smoothly and that the product is formed in high yield.
During the synthesis process, John must also use his knowledge of σ and π-bonds to predict how the molecule will react at each step. For example, he may need to protect certain functional groups to prevent them from reacting prematurely, or he may need to activate other groups to facilitate a particular reaction.
Once John has synthesized the molecule, he must analyze its properties to ensure that it is the desired compound. He does this using a variety of techniques, such as NMR spectroscopy and mass spectrometry. By analyzing the spectral data, he can confirm that the molecule contains the correct functional groups, and that it is free from impurities.
Overall, John’s work demonstrates the importance of understanding the basic principles of organic chemistry, including σ and π-bonds, in designing and synthesizing new molecules for medical applications. By applying his knowledge of chemical bonding and reactivity, John is able to design and synthesize a molecule that has the potential to be a more effective treatment for a particular disease.
White paper on Basic Principles of Organic Chemistry σ and π-bonds
Here is a white paper that provides an overview of the basic principles of organic chemistry, including σ and π-bonds:
Introduction
Organic chemistry is the study of carbon-based compounds and their reactions. Understanding the basic principles of organic chemistry is essential for a wide range of applications, including drug discovery, materials science, and environmental science. In this white paper, we will discuss the basic principles of organic chemistry, with a focus on σ and π-bonds.
Carbon-Based Compounds
Carbon-based compounds are the foundation of organic chemistry. Carbon is unique among the elements in its ability to form strong covalent bonds with other carbon atoms, as well as with other elements such as hydrogen, oxygen, nitrogen, and sulfur. These bonds are the basis for the rich and diverse chemistry of organic compounds.
Chemical Bonding
Chemical bonding is the process by which atoms come together to form molecules. In organic chemistry, there are two main types of chemical bonds: σ and π-bonds.
σ-Bonds
A σ-bond is a covalent bond in which the electron density is concentrated along the bond axis. These bonds are formed when two atoms share a pair of electrons in a direct head-to-head overlap of their atomic orbitals. σ-bonds are the strongest type of chemical bond and are found in all covalent compounds.
π-Bonds
A π-bond is a covalent bond in which the electron density is concentrated above and below the bond axis. These bonds are formed when two atoms share a pair of electrons in a sideways overlap of their atomic orbitals. π-bonds are weaker than σ-bonds but are still essential for the chemistry of many organic compounds.
Functional Groups
Functional groups are specific arrangements of atoms within organic molecules that are responsible for their reactivity and properties. Some common functional groups include alcohols, amines, carboxylic acids, and esters. Understanding the reactivity and properties of functional groups is essential for designing and synthesizing new organic compounds.
Synthesis and Analysis
The synthesis of organic compounds involves a series of chemical reactions that convert starting materials into desired products. Organic synthesis requires careful control of reaction conditions and an understanding of the reactivity and properties of functional groups. Analyzing the properties of organic compounds is also essential for verifying the identity and purity of synthesized compounds. Techniques such as NMR spectroscopy and mass spectrometry are commonly used to analyze the structure and composition of organic compounds.
Conclusion
In conclusion, understanding the basic principles of organic chemistry, including σ and π-bonds, is essential for a wide range of applications. These principles are the foundation for the design and synthesis of new organic compounds for use in drug discovery, materials science, and environmental science. With a solid understanding of the basic principles of organic chemistry, researchers can unlock the vast potential of carbon-based chemistry to address some of the world’s most pressing challenges.