JEE (Joint Entrance Examination) Main and Advanced are national level entrance exams conducted for admission to undergraduate engineering programs in various prestigious engineering colleges and institutes in India. The syllabus for JEE Advanced includes a variety of topics in chemistry, including carboxylic acids.
Carboxylic acids are organic compounds that contain a carboxyl group (-COOH) attached to a carbon atom. Some important topics related to carboxylic acids that are covered in JEE Advanced include:
- Nomenclature and structure: The rules for naming carboxylic acids and the structure of the carboxyl group.
- Preparation: Various methods for the preparation of carboxylic acids, including oxidation of primary alcohols, hydrolysis of nitriles, and carboxylation of Grignard reagents.
- Physical properties: The physical properties of carboxylic acids, such as boiling point, solubility, and acidity, and their relation to their molecular structure.
- Chemical reactions: The various chemical reactions of carboxylic acids, including nucleophilic substitution reactions, reduction, and decarboxylation reactions.
- Derivatives: The derivatives of carboxylic acids, such as acid chlorides, esters, and amides, and their preparation and reactions.
- Uses: The uses of carboxylic acids in industry, including as food additives, pharmaceuticals, and in the manufacture of polymers.
Overall, a thorough understanding of the chemistry of carboxylic acids is essential for success in JEE Advanced and for a career in the field of chemistry.
History of JEE (Main+Advance) Advance Courses Carboxylic Acids
The study of carboxylic acids dates back to the 18th century, when chemists such as Scheele and Lavoisier discovered and named some of the most common carboxylic acids, including acetic acid and formic acid. In the 19th century, chemists began to study the physical and chemical properties of carboxylic acids in more detail, and new methods for their synthesis and analysis were developed.
The concept of carboxylic acid derivatives, such as esters and amides, was first introduced by Liebig in the mid-19th century. This led to the development of various methods for the preparation and reactions of carboxylic acid derivatives, which are widely used today in organic chemistry.
In the early 20th century, the development of spectroscopic techniques, such as infrared and NMR spectroscopy, allowed chemists to study the structures and properties of carboxylic acids in more detail. This led to the discovery of new types of carboxylic acids and the development of new methods for their synthesis and analysis.
Today, carboxylic acids and their derivatives are widely used in various fields, including medicine, agriculture, food industry, and materials science. The study of carboxylic acids is an important part of the curriculum in chemistry and related fields, and it continues to evolve with new discoveries and applications. JEE (Main+Advance) Advance Courses cover a wide range of topics related to carboxylic acids, and a strong foundation in this area is essential for success in the field of chemistry.
Importance of JEE (Main+Advance) Advance Courses Carboxylic Acids
The study of carboxylic acids is an important part of JEE (Main+Advance) Advance Courses in chemistry, as it is essential for understanding many aspects of organic chemistry. Here are some reasons why carboxylic acids are important:
- They are important biological molecules: Carboxylic acids are found in many biological systems, such as the citric acid cycle, which is the process that produces energy in living cells. Understanding the properties and reactions of carboxylic acids is essential for understanding many aspects of biochemistry and molecular biology.
- They are important industrial chemicals: Many carboxylic acids and their derivatives are used in various industries, such as food, pharmaceuticals, and materials science. The study of carboxylic acids is essential for the design and synthesis of new materials and for the development of new industrial processes.
- They are versatile building blocks: Carboxylic acids are versatile building blocks in organic synthesis and can be used to make a wide range of other organic compounds, including esters, amides, and acid chlorides. A good understanding of the chemistry of carboxylic acids is essential for success in organic synthesis.
- They are involved in many chemical reactions: Carboxylic acids are involved in a wide range of chemical reactions, including acid-base reactions, nucleophilic substitution reactions, and reduction reactions. Understanding these reactions and their mechanisms is essential for success in JEE (Main+Advance) Advance Courses and for a career in chemistry.
Overall, the study of carboxylic acids is essential for understanding many aspects of organic chemistry, and a good understanding of this topic is essential for success in JEE (Main+Advance) Advance Courses in chemistry and for a career in the field of chemistry.
Benefits of JEE (Main+Advance) Advance Courses Carboxylic Acids
JEE (Joint Entrance Examination) Main and Advanced are national level entrance exams conducted for admission to undergraduate engineering programs in various prestigious engineering colleges and institutes in India. The study of carboxylic acids in JEE (Main+Advance) Advance Courses in chemistry offers several benefits for students pursuing a career in chemistry or related fields. Here are some benefits of studying carboxylic acids in JEE (Main+Advance) Advance Courses:
- Understanding the chemistry of biological systems: Carboxylic acids are essential components of many biological systems, such as the citric acid cycle, which is the process that produces energy in living cells. Understanding the properties and reactions of carboxylic acids is essential for understanding many aspects of biochemistry and molecular biology.
- Preparation for a career in industry: Many carboxylic acids and their derivatives are used in various industries, such as food, pharmaceuticals, and materials science. Studying carboxylic acids in JEE (Main+Advance) Advance Courses provides students with the knowledge and skills necessary to design and synthesize new materials and develop new industrial processes.
- Development of synthetic skills: Carboxylic acids are versatile building blocks in organic synthesis and can be used to make a wide range of other organic compounds, including esters, amides, and acid chlorides. Studying carboxylic acids in JEE (Main+Advance) Advance Courses provides students with the knowledge and skills necessary to succeed in organic synthesis and develop new synthetic routes.
- Preparation for further studies: Carboxylic acids are an essential part of the foundation of organic chemistry. Studying carboxylic acids in JEE (Main+Advance) Advance Courses provides students with a strong foundation in organic chemistry, which is essential for success in further studies in chemistry or related fields.
- Career opportunities: A strong understanding of carboxylic acids and their chemistry provides students with a wide range of career opportunities, including in the pharmaceutical industry, the food industry, materials science, and many other fields.
Overall, studying carboxylic acids in JEE (Main+Advance) Advance Courses provides students with a strong foundation in organic chemistry, which is essential for success in further studies and in a career in chemistry or related fields.
Overview of JEE (Main+Advance) Advance Courses Carboxylic Acids
JEE (Joint Entrance Examination) Main and Advanced are national level entrance exams conducted for admission to undergraduate engineering programs in various prestigious engineering colleges and institutes in India. The syllabus for JEE Advanced includes a variety of topics in chemistry, including carboxylic acids. Here is a brief overview of the JEE (Main+Advance) Advance Courses on carboxylic acids:
- Introduction: The introduction to carboxylic acids, their nomenclature, and structure.
- Physical properties: The physical properties of carboxylic acids, such as boiling point, solubility, and acidity, and their relation to their molecular structure.
- Chemical reactions: The various chemical reactions of carboxylic acids, including acid-base reactions, nucleophilic substitution reactions, reduction reactions, and decarboxylation reactions.
- Preparation: Various methods for the preparation of carboxylic acids, including oxidation of primary alcohols, hydrolysis of nitriles, and carboxylation of Grignard reagents.
- Derivatives: The derivatives of carboxylic acids, such as acid chlorides, esters, and amides, and their preparation and reactions.
- Uses: The uses of carboxylic acids in industry, including as food additives, pharmaceuticals, and in the manufacture of polymers.
The JEE (Main+Advance) Advance Courses on carboxylic acids also cover related topics, such as the reactions of carboxylic acids with alcohols to form esters and the reactions of carboxylic acids with amines to form amides.
Overall, the JEE (Main+Advance) Advance Courses on carboxylic acids provide students with a strong foundation in the chemistry of carboxylic acids, which is essential for success in further studies in chemistry or related fields and in a career in the chemical industry.
Application in Life Sciences of JEE (Main+Advance) Advance Courses Carboxylic Acids
Carboxylic acids are compounds happening normally in various phases of life cycles (living organic entity Krebs cycle; maturation processes, and topographical cycles) or can be delivered in the research centers or at large scale (combination) from oxidation responses of aldehydes, essential alcohols, and hydrocarbons, oxidative cleavage of olefins, base catalyzed dehydrogenation of alcohols or through the hydrolysis of nitriles, esters, or amides. The natural acids assume huge and differed parts in our contemporary society as confirmed by various applications in the field of medication, agribusiness, drugs, food, and different businesses.
Carboxylic acids and their subsidiaries are utilized in the creation of polymers, biopolymers, coatings, glues, and drug drugs. They additionally can be utilized as solvents, food added substances, antimicrobials, and flavorings.
Natural acids play significant parts in the food business, since they influence the organoleptic properties (for example taste, fragrance, and variety) and the soundness of food things. They can be available as normal food parts, for instance, the acids present in leafy foods (citrus extract in citrus natural products, malic corrosive in grapes and apples, oxalic corrosive salts in parsley, broccoli), or added misleadingly, as acidulants (citrus extract), additives (lactic corrosive), emulsifiers (tartaric corrosive), cell reinforcements (ascorbic corrosive), or flavors (propionic corrosive) in a wide assortment of items for human utilization (food varieties and refreshments). The level and nature of natural acids present in food varieties and beverages give significant data to checking the maturation processes, control the creation, stockpiling, and appropriation arranges or distinguish conceivable defilement activities. Exactly for this reason, logical techniques should be ceaselessly evolved and applied to recognize and measure the measures of various acids present in food and refreshments. The refreshment business (squeezes and beverages) is perhaps of the most controlled and directed industry with regards to organization and validness of items. Natural acids are notable as viable additives, and their antimicrobial activity is because of the capacity to change from undissociated to separated structure, contingent upon the ecological pH, making them powerful antimicrobial specialists. A model, a few natural salts (calcium and sodium propionate) forestall deterioration by hindering the development of microscopic organisms and parasites and are utilized as additives in dairy and pastry kitchen food items. Notwithstanding, there are carboxylic acids that usefully affect miniature organic entities, helping their development by going about as nutrients for microbial nourishment (for example folic corrosive, nicotinic corrosive, or p-aminobenzoic corrosive). A few examinations on the inhibitory impact of different natural acids (oxalic, citrus, and malic acids) on polyphenol oxidase (PPO), the chemical liable for the sautéing of harmed leafy foods, have been led throughout the long term. The successes of these examinations have had a positive financial effect in the food business, where keeping up with the quality and expanding the items time span of usability addresses a need.
Carboxylic acids likewise assume critical parts in the restorative fields. Since natural acids are moderate metabolites of all significant gatherings of natural cell parts, it has been over and over demonstrated that their presence in abundance in different liquids of the human body is connected to the appearance of specific illnesses. Natural acids are marks of natural acidurias related with different characteristic mistakes of protein digestion. In excess of 65 issues notable these days are because of chemical lack in the amino acids debasement pathways (leucine, isoleucine, valine, homocysteine, tyrosine, methionine, threonine, lysine, and tryptophan) bringing about an increment of the natural corrosive fixation available for use or discharged pee. This harmful aggregation of metabolites, which are absent under physiological circumstances in the life form, causes an inebriation like clinical condition. The urinary natural corrosive example yielded from these metabolic irregularities is fundamental for conclusion. For instance, the degrees of homovanillic corrosive (HVA) and vanillylmandelic acids (VMAs) in body liquids are utilized in the conclusion of neurological sicknesses and issues. Investigations of the metabolic fingerprints partner the option less effective debasement pathways of unsaturated fats (prompting expanded degrees of adipic and sub Eric acids in urinary discharge) with messes like mental imbalance. The degrees of succinic corrosive in clinical examples demonstrate the event of a bacterial disease without the chance of separation among high-impact and anaerobic microbes type. The measurement of natural acids levels in body liquids can give helpful data in basic areas of digestion: synapse digestion, gastrointestinal capability, cell energy and mitochondrial digestion, and amino corrosive/natural corrosive offset with the motivation behind an early conclusion of different sicknesses.
The drug business benefits too from the presence and the elements of carboxylic acids. Making sense of the significance of carboxylic acids and their subsidiaries in the drug business depend on the synthetic idea of the practical gathering. The main jobs that carboxylic capabilities play in drugs are:
Solubilize acting in regulating solvency, lipophilicity, and cell saturation (for example anti-toxin or antihistaminic drug classes);
Prodrug as well as bioprecursor going about as mixtures not organically dynamic yet changed over into dynamic ones in unambiguous circumstances (for example drugs from antihypertensive, antithrombotic, or antiviral classes);
Pharmacophore giving explicit collaborations a catalyst, setting off, or impeding its natural reaction (for example blood cholesterol-lessening drugs, nonsteroidal mitigating drugs).
Carboxylic corrosive containing drugs assume a significant part in the clinical treatment of torment and illnesses.
They are likewise utilized in a wide assortment of uses as fixings in beauty care products. A class of natural acids with a significant commitment in the restorative field is the alpha hydroxy acids (AHAs). Citrus, malic, tartaric, and lactic and glycolic acids are essential for this classification and are broadly utilized in beauty care products for purposes such unblock/clean pores, further develop the skin surface, brightening, against kink, or skin break out treatment. Likewise, carboxylic acids addressed by aldobionic acids (ABAs), retinoic acids, L-ascorbic acid, and azelaic corrosive are best in giving cell reinforcement and against maturing security, as well as further developing dampness maintenance. The carboxylic corrosive based esters are the subordinates generally notable for their flavors and scents and are broadly utilized in different applications including aromas, antiperspirant, and deodorizers.
Unsaturated fats address the class of carboxylic acids perceived for its utility in the corrective business since their water-solvent salts (cleansers) have been utilized as cleaning agents, since relic and are the most helpful surfactants known.
In spite of the fact that is a debate issue about the job of natural acids in land processes, an assortment of studies and information give bits of knowledge and examination bearings concerning their effect on geographical cycles. Many reports proposed that natural acids have taken part in soil development, surface enduring, subsurface porosity age, and mineral arrangement. Additionally, concentrates on depicted the huge and fluctuated jobs of natural acids played in rhizosphere fermentation and mineral enduring. Writing information list the significant jobs of natural acids in rhizosphere cycles like preparation of supplements (Fe, phosphates), assurance against aluminum harmfulness, expanding the enduring pace of essential minerals, and taking part in movement of Fe and Al. Different classes of carboxylic acids, for example, phenolic and unsaturated fats repress the ordinary development of plants and green growth, going about as allelochemicals, bringing about unfavorable effects on the physiological and biological climate (changed microflora).
One part of this book offers, the itemized conversation of systems of natural corrosive on the procurement of soil phosphate in the fields of plant physiology, plant nourishment, and soil science. Some plant species firmly activate soil phosphate via carboxylates further developing this macronutrient procurement.
Conclusion of JEE (Main+Advance) Advance Courses Carboxylic Acids
The carboxylic corrosive mixtures actually may find applications that can’t be completely canvassed in this section. As end, beginning from food to medication, from the human body to earth and climate, the creation, obliteration, ingestion, or arrival of these mixtures show serious areas of strength for an on every one of the cycles/responses that happen.
As a last end, this subject is a perpetual one and the classes of mixtures that contain the carboxyl practical gathering, alongside the entirety of their subsidiaries, are indivisible from all that life implies on this planet.
Structures of JEE (Main+Advance) Advance Courses Carboxylic Acids
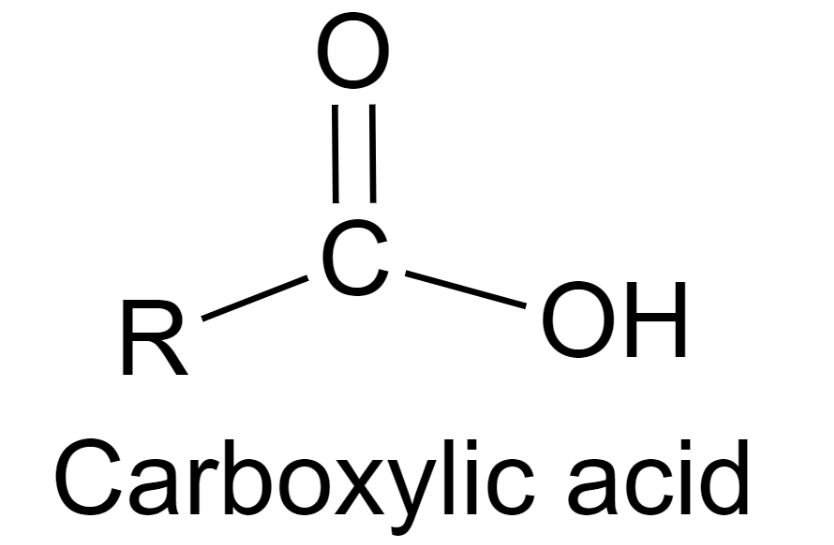
The overall equation of a carboxylic corrosive is R-COOH, where COOH alludes to the carboxyl gathering, and R alludes to the remainder of the particle to which this gathering is connected. In this carboxyl gathering, there exists a carbon what imparts a twofold cling to an oxygen molecule and a solitary bond with a hydroxyl bunch.
A carboxylic corrosive’s overall equation is R-COOH, where COOH indicates the carboxyl gathering and R means the rest of the particle to which this gathering is connected. There is a carbon in this carboxyl gathering that has a twofold association with an oxygen molecule and a solitary bond with a hydroxyl bunch.
The initial four carboxylic acids got from alkanes are methanoic corrosive (HCOOH), ethanoic corrosive (CH3COOH), propanoic corrosive (C2H5COOH), and butanoic corrosive (C3H7COOH).
The general design of a carboxylic corrosive is represented underneath.
From the representation gave above, it tends to be seen that a carboxylic corrosive contains a hydroxyl bunch connected to a carbonyl carbon. Because of the electronegativity of the oxygen iota, this utilitarian gathering can go through ionization and release a proton.
The carboxylate particle, created from the expulsion of a proton from the carboxyl gathering, is settled by the presence of two oxygen molecules (through which the negative charge can move). A few normal instances of carboxylic acids incorporate acidic corrosive (a part of vinegar) and Formic corrosive.
Nomenclature of JEE (Main+Advance) Advance Courses Carboxylic Acids
By and large, these natural mixtures are alluded to by their minor names, which contain the postfix “- ic corrosive”. An illustration of an inconsequential name for a carboxylic corrosive is acidic corrosive (CH3COOH). In the IUPAC terminology of these mixtures, the addition “- oic corrosive” is relegated.
The rules that should be continued in the IUPAC classification of carboxylic acids are recorded underneath.
The postfix “e” for the sake of the relating alkane is supplanted with “oic corrosive”.
At the point when the aliphatic chain contains just a single carboxyl gathering, the carboxylic carbon is constantly numbered one. For instance, CH3COOH is named as ethanoic corrosive.
At the point when the aliphatic chain contains more than one carboxyl gathering, the all out number of carbon particles is counted and the quantity of carboxyl gatherings is addressed by Greek numeral prefixes, for example, “di-“, “tri-“, and so on.
A carboxylic corrosive is named by adding these prefixes and postfixes to the parent alkyl chain. Arabic numerals are utilized for showing the places of the carboxyl gathering.
The name “carboxylic corrosive” or “carboxy” can likewise be doled out for a carboxyl substituent on a carbon chain. An illustration of such terminology is the name 2-carboxyfuran for the compound 2-Furoic corrosive.
Career Opportunities of JEE (Main+Advance) Advance Courses Carboxylic Acids
There are several career opportunities available for individuals who have a strong understanding of Carboxylic Acids, which is covered in the JEE (Main+Advance) Advance Courses. Here are some potential career paths:
- Chemical Research: Carboxylic acids play a crucial role in chemical research, and individuals who have a strong understanding of this topic can pursue careers in research and development. This could include working in research and development departments in various industries such as pharmaceuticals, food and beverage, and biotechnology.
- Environmental Science: Carboxylic acids are also relevant in environmental science, where they are studied in relation to pollution, waste management, and climate change. A strong understanding of carboxylic acids can lead to careers in environmental research, analysis, and policy.
- Organic Chemistry: Carboxylic acids are a fundamental part of organic chemistry, and individuals with a strong grasp of this topic can pursue careers in various industries such as petrochemicals, plastics, and pharmaceuticals. They could work in research and development departments or as chemists in manufacturing facilities.
- Agriculture: Carboxylic acids play an important role in agriculture, where they are used as pesticides and herbicides. Individuals with knowledge of carboxylic acids could work in agricultural research, developing new products or improving existing ones.
- Academia: Those with a deep understanding of carboxylic acids could pursue careers in academia, teaching and conducting research in chemistry, biology, and related fields.
Overall, a strong understanding of carboxylic acids can lead to various career opportunities in research, development, and academia, among others.
