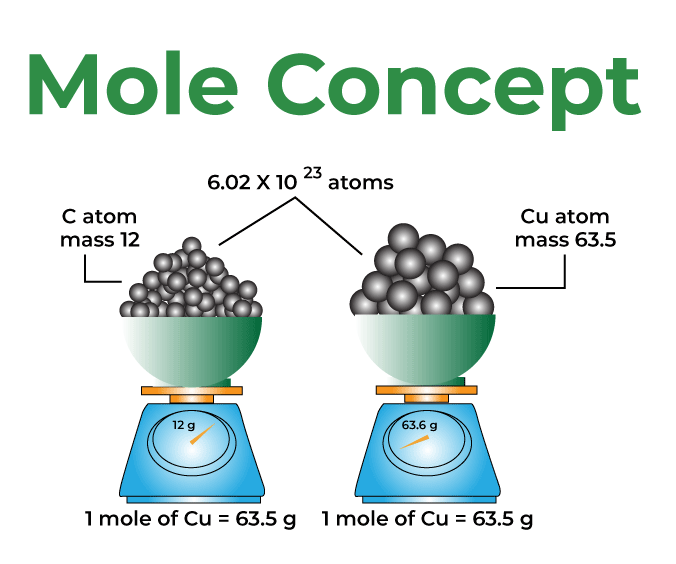
The mole concept is a fundamental concept in chemistry that allows chemists to measure and relate quantities of substances. The concept is based on the idea that atoms, molecules, and other particles are very small and difficult to count on an individual basis, so they are instead measured in terms of the amount of substance they make up.
One mole of a substance is defined as the amount of that substance that contains the same number of particles as there are atoms in 12 grams of carbon-12. This number is known as Avogadro’s number and is approximately 6.022 x 10^23.
The mole concept is used in a variety of ways in chemistry, such as in stoichiometry to calculate the amounts of reactants and products in a chemical reaction, in determining the concentration of a solution, and in measuring the mass of a substance in grams per mole.
Understanding the mole concept is essential for anyone studying chemistry, as it provides a basic framework for understanding the relationships between different substances and their properties.
What is Required Mole concept
The Required Mole Concept in chemistry refers to the use of the mole unit to perform various calculations related to chemical reactions and stoichiometry. The Required Mole Concept allows chemists to determine the quantity of reactants needed for a given reaction, the quantity of products produced, and the amount of excess or limiting reactants.
To use the Required Mole Concept, one needs to know the balanced chemical equation for the reaction, which indicates the stoichiometry, or relative quantities, of reactants and products. The coefficients in the balanced equation represent the number of moles of each substance involved in the reaction.
Once the balanced equation is known, the Required Mole Concept can be used to calculate the amount of reactants or products needed or produced, based on the mole ratios of the substances in the reaction. For example, if one knows the number of moles of one substance, they can use the mole ratios in the balanced equation to determine the number of moles of another substance involved in the reaction.
The Required Mole Concept is an important tool in chemistry, as it allows chemists to accurately predict the amount of product that will be produced in a chemical reaction, as well as to optimize reaction conditions by ensuring that the reactants are present in the correct proportions.
Who is Required Mole concept
“Required Mole Concept” is not a person, it is a concept in chemistry that involves the use of the mole unit to perform calculations related to chemical reactions and stoichiometry. The mole concept is named after the Italian scientist Amedeo Avogadro, who in the early 19th century proposed that equal volumes of gases at the same temperature and pressure contain the same number of particles, now known as Avogadro’s law. This led to the development of the concept of the mole, which is defined as the amount of a substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. The mole concept is a fundamental concept in chemistry and is widely used in various applications, including the calculation of reactant and product amounts, the determination of molar mass, and the calculation of concentration in solutions.
When is Required Mole concept
The Required Mole Concept is used in chemistry whenever quantities of substances need to be related to each other, such as in chemical reactions, stoichiometry, and solution chemistry. The concept is used to convert between mass, volume, and number of particles of a substance, as well as to calculate the amount of reactants or products needed or produced in a chemical reaction.
The Required Mole Concept is particularly useful when dealing with chemical equations, as it allows chemists to determine the stoichiometry, or relative quantities, of reactants and products involved in a reaction. By using the coefficients in the balanced equation, chemists can determine the number of moles of each substance involved in the reaction, and then use this information to calculate the amounts of reactants needed or products produced.
The Required Mole Concept is also used in solution chemistry to calculate the concentration of a solution in terms of moles per liter. By knowing the amount of solute in moles and the volume of the solution, chemists can calculate the molarity of the solution.
In summary, the Required Mole Concept is used in a variety of applications in chemistry whenever quantities of substances need to be related to each other. It is an essential concept for anyone studying chemistry and is used in a wide range of chemical calculations.
Where is Required Mole concept
The Required Mole Concept is used in various areas of chemistry, including in chemical reactions, stoichiometry, solution chemistry, and many other fields.
In chemical reactions, the Required Mole Concept is used to determine the quantities of reactants and products involved in the reaction, based on the balanced chemical equation. Chemists can use the mole ratios in the equation to calculate the amounts of reactants needed or products produced, and to determine which reactant is the limiting reactant and which is in excess.
In stoichiometry, the Required Mole Concept is used to calculate the amount of a substance needed or produced in a chemical reaction, based on the mole ratios in the balanced chemical equation. This allows chemists to determine the theoretical yield of a reaction, as well as to calculate the percent yield and the amount of excess or limiting reactants.
In solution chemistry, the Required Mole Concept is used to calculate the concentration of a solution in terms of moles per liter. This is known as the molarity of the solution, and it is an important parameter in many chemical processes.
Overall, the Required Mole Concept is a fundamental concept in chemistry that is used in many different areas of the field. It allows chemists to relate the quantities of substances to each other and to perform calculations related to chemical reactions and stoichiometry.
How is Required Mole concept
The Required Mole Concept is used in chemistry to relate the amount of a substance to its molecular or atomic weight, and to calculate the amount of reactants needed or products produced in a chemical reaction.
The concept is based on the mole unit, which is defined as the amount of a substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. The mole unit allows chemists to relate the mass of a substance to the number of particles it contains, and vice versa.
To use the Required Mole Concept, one needs to know the balanced chemical equation for the reaction, which indicates the stoichiometry, or relative quantities, of reactants and products. The coefficients in the balanced equation represent the number of moles of each substance involved in the reaction.
Once the balanced equation is known, chemists can use the mole ratios in the equation to calculate the amounts of reactants needed or products produced. For example, if one knows the number of moles of one substance, they can use the mole ratios in the balanced equation to determine the number of moles of another substance involved in the reaction.
In addition to calculating the amounts of reactants and products in a chemical reaction, the Required Mole Concept is also used to calculate the molar mass of a substance, which is the mass of one mole of the substance. This is important for determining the amount of a substance present in a sample, as well as for calculating the concentration of a solution in terms of moles per liter.
In summary, the Required Mole Concept is used in chemistry to relate the amount of a substance to its molecular or atomic weight, and to calculate the amount of reactants needed or products produced in a chemical reaction. The concept is based on the mole unit, which allows chemists to relate the mass of a substance to the number of particles it contains, and vice versa.
Case Study on Mole concept
Case Study: Calculating the Amount of Reactants Required for a Chemical Reaction
A chemical reaction takes place between sodium hydroxide (NaOH) and hydrochloric acid (HCl) to produce sodium chloride (NaCl) and water (H2O). The balanced chemical equation for the reaction is:
NaOH + HCl → NaCl + H2O
Suppose we want to carry out this reaction and we have 25 mL of 0.1 M HCl solution. We want to know how much NaOH we need to add to react with all of the HCl and produce the maximum amount of NaCl possible.
To solve this problem using the mole concept, we need to first convert the volume and concentration of the HCl solution into the number of moles of HCl. We can use the formula:
moles = concentration (M) x volume (L)
Since we have 25 mL of 0.1 M HCl solution, the number of moles of HCl is:
moles of HCl = 0.1 M x 0.025 L = 0.0025 moles of HCl
Now that we know the number of moles of HCl, we can use the mole ratio in the balanced equation to determine the number of moles of NaOH required to react with all of the HCl. From the balanced equation, we can see that the mole ratio of NaOH to HCl is 1:1, which means that we need the same number of moles of NaOH as we have moles of HCl.
So, we need 0.0025 moles of NaOH to react with all of the HCl. To convert this to mass, we need to know the molar mass of NaOH, which is 40 g/mol. Therefore, the mass of NaOH required is:
mass of NaOH = moles of NaOH x molar mass of NaOH mass of NaOH = 0.0025 moles x 40 g/mol mass of NaOH = 0.1 g
So, we need 0.1 g of NaOH to react with all of the HCl and produce the maximum amount of NaCl possible. This calculation is based on the mole concept, which allows us to relate the amount of reactants to their molecular weights and to determine the stoichiometry of the reaction using the mole ratio in the balanced equation.
White paper on Mole concept
Introduction
The mole concept is a fundamental concept in chemistry that allows chemists to relate the mass of a substance to the number of entities (atoms, molecules, ions, etc.) it contains. The mole unit is used to quantify the amount of a substance and is defined as the amount of a substance that contains the same number of entities as there are atoms in 12 grams of carbon-12. In this white paper, we will discuss the mole concept and its applications in chemistry.
The Mole Unit
The mole unit allows chemists to relate the mass of a substance to the number of entities it contains. The Avogadro’s number (6.022 x 10^23) represents the number of entities in one mole of a substance. For example, one mole of water (H2O) contains 6.022 x 10^23 molecules of water.
The mole unit also allows chemists to determine the mass of one mole of a substance, which is called the molar mass. The molar mass is expressed in grams per mole (g/mol) and is equal to the atomic or molecular weight of a substance. For example, the molar mass of water is 18.015 g/mol, which is equal to the sum of the atomic weights of two hydrogen atoms and one oxygen atom.
Applications of the Mole Concept
The mole concept has several applications in chemistry, including:
- Calculating the amount of reactants required for a chemical reaction: The mole concept is used to determine the stoichiometry of a chemical reaction and to calculate the amount of reactants required to produce a certain amount of product.
- Calculating the amount of product produced in a chemical reaction: The mole concept is used to calculate the amount of product produced in a chemical reaction based on the amount of reactants used and the stoichiometry of the reaction.
- Determining the concentration of a solution: The mole concept is used to calculate the concentration of a solution in terms of moles per liter (mol/L) or molarity.
- Determining the empirical and molecular formulas of a compound: The mole concept is used to determine the empirical and molecular formulas of a compound based on its elemental composition.
- Calculating the percentage composition of a compound: The mole concept is used to calculate the percentage composition of a compound based on its elemental composition.
Conclusion
The mole concept is a fundamental concept in chemistry that allows chemists to relate the mass of a substance to the number of entities it contains. The mole unit is used to quantify the amount of a substance and is defined as the amount of a substance that contains the same number of entities as there are atoms in 12 grams of carbon-12. The mole concept has several applications in chemistry, including calculating the amount of reactants required for a chemical reaction, determining the concentration of a solution, and calculating the empirical and molecular formulas of a compound.
