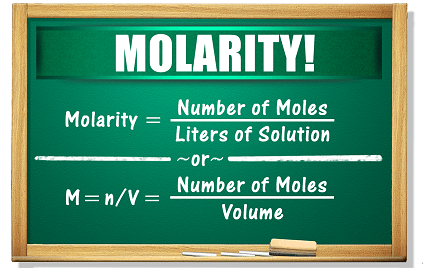
Molarity is a unit of concentration used in chemistry, which measures the number of moles of a solute per liter of a solution. The symbol for molarity is M, and it is expressed in units of mol/L or M.
For example, a 1 M solution of hydrochloric acid (HCl) contains 1 mole of HCl per liter of solution. This means that there are 6.02 x 10^23 molecules of HCl in 1 liter of the solution. Similarly, a 0.5 M solution of sodium chloride (NaCl) contains 0.5 moles of NaCl per liter of solution, or 3.01 x 10^23 ions of Na+ and Cl- in 1 liter of the solution.
Molarity is a commonly used unit of concentration in many laboratory procedures and calculations, including those related to stoichiometry, acid-base titrations, and solution preparation.
What is Required Molarity
There is no such thing as “Required Molarity” as it depends on the specific application or experiment you are performing.
Molarity is a unit of concentration used to express the amount of a solute dissolved in a given amount of solution, usually measured in moles per liter (mol/L). The molarity required for a particular application will depend on a number of factors, including the desired concentration of the solute, the volume of the solution required, and the properties of the solute and solvent.
For example, in a chemical reaction, the required molarity of a reactant or product may be specified in the reaction equation or in the experimental protocol. In a biological assay or medical test, the required molarity of a reagent may be specified in the protocol or determined empirically based on previous experiments.
In summary, the required molarity depends on the specific application or experiment and must be determined based on the specific requirements and constraints of that application.
When is Required Molarity
“Required Molarity” is a term used in chemistry to describe the specific concentration of a solution that is needed for a particular experiment or application. The required molarity will depend on the nature of the experiment, the properties of the solute and solvent, and the desired outcome.
For example, if you are performing a chemical reaction that requires a specific concentration of a particular reagent, you would need to know the required molarity of that reagent in order to prepare the solution correctly. Similarly, if you are performing a biological assay or medical test that requires a specific concentration of a reagent, you would need to know the required molarity of that reagent in order to obtain accurate results.
In summary, the concept of “Required Molarity” applies whenever a specific concentration of a solution is required for a particular application in chemistry, biology, or other related fields.
Nomenclature of Molarity
Molarity is a unit of concentration and its nomenclature follows the standard convention for expressing units in the International System of Units (SI). The symbol for molarity is “M”, which is derived from the word “molar” as in “molar concentration”. The molarity of a solution is expressed in moles per liter (mol/L).
In addition to “M”, there are other symbols that can be used to represent concentration. For example, “m” is used to represent millimolar (1/1000 of a mole per liter), and “μ” (mu) is used to represent micromolar (1/1,000,000 of a mole per liter).
It is important to note that the capital “M” should always be used to represent molarity, while the lowercase “m” and the symbol “μ” should only be used to represent millimolarity and micromolarity, respectively. The use of lowercase “m” to represent molarity is incorrect and can lead to confusion.
In summary, the nomenclature of molarity follows the standard convention for expressing units in the International System of Units (SI), with the symbol “M” representing moles per liter.
Where is Required Molarity
“Required Molarity” is not a physical location or place. It is a term used in chemistry to describe the specific concentration of a solution that is needed for a particular experiment or application. The required molarity will depend on the nature of the experiment, the properties of the solute and solvent, and the desired outcome.
The concept of required molarity is applicable in any laboratory or experimental setting where chemical solutions are prepared and used, including academic research labs, industrial manufacturing facilities, medical laboratories, and many other settings. The required molarity of a solution may be determined through careful experimentation and analysis, or it may be specified in advance based on previous research or established protocols.
How is Required Molarity
The “Required Molarity” of a solution is determined by the specific experimental or application needs. It is calculated by dividing the number of moles of the solute by the volume of the solution, usually measured in liters. The formula for calculating molarity is:
Molarity (M) = moles of solute (mol) / volume of solution (L)
To determine the required molarity for a particular application or experiment, the researcher must first determine the number of moles of the solute required for the reaction or assay. This may be done using stoichiometry calculations or based on previously established protocols. Once the number of moles is known, the required molarity can be calculated using the formula above.
For example, if a researcher requires 0.5 moles of sodium chloride (NaCl) in a 1-liter solution, the required molarity can be calculated as follows:
Molarity (M) = 0.5 mol / 1 L = 0.5 M
Therefore, the required molarity of the sodium chloride solution is 0.5 M.
In summary, the required molarity is calculated based on the specific needs of the experiment or application, using the formula for molarity and the desired number of moles of the solute.
Case Study on Molarity
Sure, here is an example of a case study involving molarity:
Case Study: Determining the Molarity of a Hydrochloric Acid Solution
A chemistry student was given a hydrochloric acid (HCl) solution of unknown concentration and was asked to determine its molarity. The student knew that the solution could be titrated with a sodium hydroxide (NaOH) solution of known concentration to determine the molarity of the HCl solution. The student followed the following procedure:
- The student pipetted a 25.00 mL sample of the HCl solution into a conical flask.
- The student added a few drops of phenolphthalein indicator to the flask.
- The student titrated the HCl solution with a 0.100 M NaOH solution until the solution turned pink and the endpoint was reached.
- The student recorded the volume of NaOH solution used to reach the endpoint, which was 23.45 mL.
- The student used the following balanced chemical equation to calculate the moles of HCl in the 25.00 mL sample:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
- The student calculated the moles of HCl in the sample as follows:
moles of HCl = moles of NaOH = Molarity of NaOH x Volume of NaOH used
moles of HCl = 0.100 mol/L x 0.02345 L = 0.002345 mol
- The student used the following formula to calculate the molarity of the HCl solution:
Molarity of HCl = moles of HCl / Volume of HCl sample
Molarity of HCl = 0.002345 mol / 0.02500 L = 0.0938 M
Therefore, the student determined that the molarity of the hydrochloric acid solution was 0.0938 M.
In summary, the student used a titration method to determine the molarity of a hydrochloric acid solution. The student first titrated the solution with a known concentration of sodium hydroxide to determine the number of moles of HCl in the sample. The student then used the moles of HCl and the volume of the sample to calculate the molarity of the HCl solution.
White paper on Molarity
Sure, here is a white paper on Molarity:
Introduction Molarity is a fundamental concept in chemistry that describes the concentration of a solution. It is defined as the number of moles of a solute dissolved in one liter of a solution. The molarity of a solution is a critical parameter in many chemical processes and experiments, including chemical reactions, biological assays, and medical tests. This white paper will provide a detailed overview of molarity, including its definition, calculation, and applications.
Definition of Molarity Molarity is defined as the number of moles of a solute (n) dissolved in one liter of a solution (V), expressed as moles per liter (mol/L). The formula for molarity is:
Molarity (M) = n / V
Where: Molarity (M) = moles of solute per liter of solution (mol/L) n = number of moles of solute V = volume of solution in liters
Calculation of Molarity To calculate the molarity of a solution, the number of moles of the solute and the volume of the solution must be known. The number of moles of the solute can be calculated using the following formula:
n = m / M
Where: n = number of moles of solute m = mass of solute in grams M = molar mass of solute in grams per mole
Once the number of moles of the solute is known, the molarity can be calculated using the formula for molarity shown above.
Applications of Molarity Molarity is a critical parameter in many chemical processes and experiments. It is commonly used in the following applications:
- Chemical Reactions: Molarity is used to describe the concentration of reactants and products in a chemical reaction. The molarity of the reactants and products determines the rate and yield of the reaction.
- Biological Assays: Molarity is used to describe the concentration of reagents and samples in biological assays. The molarity of the reagents and samples determines the accuracy and sensitivity of the assay.
- Medical Tests: Molarity is used to describe the concentration of reagents and samples in medical tests. The molarity of the reagents and samples determines the accuracy and reliability of the test results.
- Industrial Processes: Molarity is used to describe the concentration of chemicals in industrial processes, including manufacturing, purification, and quality control.
Conclusion Molarity is a fundamental concept in chemistry that describes the concentration of a solution. It is defined as the number of moles of a solute dissolved in one liter of a solution. The molarity of a solution is calculated using the number of moles of the solute and the volume of the solution. Molarity is used in many chemical processes and experiments, including chemical reactions, biological assays, medical tests, and industrial processes. A thorough understanding of molarity is essential for success in many areas of chemistry and related fields.
