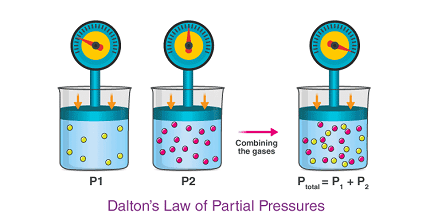
The law of partial pressures, also known as Dalton’s law, states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture.
In other words, if you have a gas mixture made up of two or more gases, the total pressure of the mixture is the sum of the pressures of each gas if it were the only gas present in the same volume and temperature.
This law is particularly useful in many areas of science and engineering, such as in the study of gases, the design of chemical processes, and in the operation of engines and turbines. It also plays a critical role in the understanding of the behavior of gases in the atmosphere and in the study of gas exchange in biological systems.
What is Required Law of partial pressures
I believe you may be referring to the Ideal Gas Law, which is a fundamental law that describes the relationship between the pressure, volume, temperature, and amount of a gas in a closed system. The Ideal Gas Law is expressed mathematically as:
PV = nRT
Where P is the pressure of the gas, V is its volume, n is the number of moles of gas present, R is the ideal gas constant, and T is the absolute temperature of the gas.
The Law of Partial Pressures, also known as Dalton’s Law, is often used in conjunction with the Ideal Gas Law to describe the behavior of gas mixtures. The Law of Partial Pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases in the mixture.
So, in order to fully describe the behavior of a gas mixture using the Ideal Gas Law, we need to take into account both the total pressure of the mixture and the partial pressures of each individual gas present in the mixture.
When is Required Law of partial pressures
The Law of Partial Pressures, also known as Dalton’s Law, is applicable whenever you have a mixture of gases or liquids. It states that the total pressure of a mixture of gases or liquids is equal to the sum of the partial pressures of the individual components in the mixture. This law is based on the fact that the individual components of a mixture exert a pressure that is proportional to their concentration in the mixture.
The Law of Partial Pressures is particularly important in the study of gases, where it is used to determine the composition of gas mixtures and to calculate the behavior of gases in various conditions. It is also used in the study of liquids, where it helps to determine the vapor pressure of a liquid mixture.
Overall, the Law of Partial Pressures is a fundamental principle in the study of gases and liquids, and it is widely used in many areas of science and engineering.
Where is Required Law of partial pressures
The Law of Partial Pressures, also known as Dalton’s Law, is a scientific principle that applies to mixtures of gases and liquids. It is a fundamental concept in the study of gases and liquids and is widely used in various fields of science and engineering.
The Law of Partial Pressures applies to any system that contains a mixture of gases or liquids, including in the atmosphere, in industrial processes, and in biological systems. For example, it can be used to calculate the composition of the air we breathe, to determine the vapor pressure of a liquid mixture in a chemical process, or to study gas exchange in the lungs.
The Law of Partial Pressures is one of the cornerstones of the study of gases and liquids, and it plays a critical role in many areas of science and engineering.
How is Required Law of partial pressures
The Law of Partial Pressures, also known as Dalton’s Law, describes the behavior of gases in a mixture. It states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture.
The Law of Partial Pressures can be understood by considering the behavior of individual gas molecules. Each gas molecule in a mixture moves independently and collides with other gas molecules. When a gas molecule collides with the walls of its container, it exerts a force, which we perceive as pressure.
The pressure of a gas is proportional to the number of gas molecules present and the force with which they collide with the container walls. In a mixture of gases, each gas exerts a partial pressure that is proportional to its concentration in the mixture. The total pressure of the mixture is simply the sum of the partial pressures of each gas.
Mathematically, the Law of Partial Pressures can be expressed as:
P_total = P_1 + P_2 + … + P_n
Where P_total is the total pressure of the gas mixture and P_1, P_2, …, P_n are the partial pressures of each gas in the mixture.
The Law of Partial Pressures is a fundamental principle in the study of gases and is used in many areas of science and engineering, including in the design of chemical processes, the study of atmospheric gases, and the operation of engines and turbines.
Why to do Law of partial pressures
The Law of Partial Pressures is important because it describes the behavior of gases in a mixture and is used in many areas of science and engineering. It helps us to understand how gases behave in various processes, such as chemical reactions, atmospheric processes, and gas engines and turbines.
By understanding the Law of Partial Pressures, we can make predictions about the behavior of gas mixtures and optimize processes to achieve desired outcomes. For example, in chemical processes, the Law of Partial Pressures can be used to predict the concentrations of reactants and products at equilibrium. In atmospheric science, it can be used to understand how different gases interact with each other and with the environment. In gas engines and turbines, it can be used to optimize performance by ensuring that the correct mixture of gases is used.
Overall, the Law of Partial Pressures is a fundamental principle in the study of gases and is essential for many applications in science and engineering.
Case Study on Law of partial pressures
Sure, I can provide a case study on the Law of Partial Pressures.
Case Study: Gas Mixture in a Chemical Process
Consider a chemical process that involves a gas mixture of nitrogen (N2) and hydrogen (H2) at a total pressure of 1 atm. The gas mixture is fed into a reactor where it reacts to form ammonia (NH3) gas.
To determine the behavior of the gas mixture in the reactor, we need to apply the Law of Partial Pressures. We know that the total pressure of the gas mixture is 1 atm, but we need to determine the partial pressures of each gas in the mixture.
Let’s assume that the mole fraction of nitrogen in the gas mixture is 0.8 and the mole fraction of hydrogen is 0.2. We can use these mole fractions to calculate the partial pressures of each gas in the mixture:
Partial pressure of nitrogen (P_N2) = mole fraction of nitrogen (0.8) x total pressure (1 atm) = 0.8 atm
Partial pressure of hydrogen (P_H2) = mole fraction of hydrogen (0.2) x total pressure (1 atm) = 0.2 atm
Now that we know the partial pressures of each gas in the mixture, we can use them to predict the behavior of the gas mixture in the reactor. Specifically, we can use the partial pressures to calculate the reaction equilibrium constant, which is a measure of the extent to which the reactants are converted to products.
The reaction that takes place in the reactor is:
N2(g) + 3H2(g) ⇌ 2NH3(g)
The equilibrium constant for this reaction is:
Kc = (P_NH3)^2 / (P_N2 x P_H2^3)
where P_NH3, P_N2, and P_H2 are the partial pressures of ammonia, nitrogen, and hydrogen, respectively.
Using the partial pressures we calculated earlier, we can determine the equilibrium constant for the reaction:
Kc = (P_NH3)^2 / (P_N2 x P_H2^3) = (x)^2 / (0.8 x 0.2^3) = x^2 / 0.00032
where x is the partial pressure of ammonia (which we don’t know yet).
Now, we can use the equilibrium constant to calculate the partial pressure of ammonia at equilibrium. Assuming that the reaction has reached equilibrium, we can write:
Kc = (P_NH3)^2 / (P_N2 x P_H2^3) = (x)^2 / (0.8 x 0.2^3) = 0.023
Solving for x, we get:
x = 0.088 atm
Therefore, the partial pressure of ammonia at equilibrium is 0.088 atm.
In summary, the Law of Partial Pressures was used to determine the behavior of a gas mixture in a chemical process. By calculating the partial pressures of each gas in the mixture and using them to determine the equilibrium constant for the reaction, we were able to predict the partial pressure of ammonia at equilibrium. This case study illustrates the importance of the Law of Partial Pressures in the design and optimization of chemical processes.
White paper on Law of partial pressures
Sure, here’s a white paper on the Law of Partial Pressures:
Introduction
The Law of Partial Pressures, also known as Dalton’s Law, is a fundamental principle in the study of gases. It describes the behavior of gases in a mixture and is used in many areas of science and engineering. In this white paper, we will discuss the Law of Partial Pressures in detail, including its history, applications, and mathematical formulation.
History
The Law of Partial Pressures is named after John Dalton, an English chemist and physicist who first proposed the concept in the early 19th century. Dalton observed that the total pressure of a mixture of gases is equal to the sum of the pressures of the individual gases in the mixture. He also proposed that the behavior of gases can be explained by assuming that they consist of small, indivisible particles called atoms.
Dalton’s Law was later refined by other scientists, including the French chemist Joseph Louis Gay-Lussac, who showed that gases combine in simple numerical ratios. Today, the Law of Partial Pressures is a cornerstone of the study of gases and is used in many areas of science and engineering.
Applications
The Law of Partial Pressures has many applications in science and engineering. Some examples include:
- Gas mixtures in chemical processes: In many chemical processes, gases are mixed together and react to form new compounds. The Law of Partial Pressures can be used to predict the behavior of gas mixtures in these processes, including the equilibrium concentrations of reactants and products.
- Atmospheric gases: The Earth’s atmosphere is composed of many different gases, including nitrogen, oxygen, carbon dioxide, and water vapor. The Law of Partial Pressures can be used to understand how these gases behave and interact with each other in the atmosphere.
- Gas engines and turbines: Gas engines and turbines convert the energy of a gas into mechanical energy. The Law of Partial Pressures is used to optimize the performance of these devices by ensuring that the correct mixture of gases is used.
Mathematical Formulation
The Law of Partial Pressures can be mathematically formulated as follows:
P_total = P_1 + P_2 + … + P_n
where P_total is the total pressure of a mixture of gases, and P_1, P_2, …, P_n are the partial pressures of each gas in the mixture.
The partial pressure of a gas is defined as the pressure that the gas would exert if it were the only gas present in the mixture. The partial pressure of a gas depends on its concentration in the mixture and the temperature and volume of the container.
The Law of Partial Pressures is based on the idea that each gas in a mixture behaves independently and exerts a pressure that is proportional to its concentration in the mixture. The total pressure of the mixture is simply the sum of the pressures exerted by each gas.
Conclusion
The Law of Partial Pressures, also known as Dalton’s Law, is a fundamental principle in the study of gases. It describes the behavior of gases in a mixture and is used in many areas of science and engineering. The Law of Partial Pressures can be mathematically formulated as the sum of the partial pressures of each gas in the mixture. Understanding the Law of Partial Pressures is essential for the design and optimization of many processes, including chemical processes, atmospheric processes, and gas engines and turbines.
