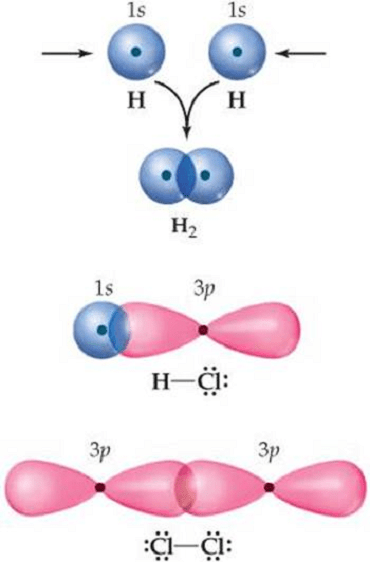
Orbital overlap is a concept in chemistry that refers to the interaction between two or more atomic orbitals when they come together to form a chemical bond. When atoms bond together, their atomic orbitals can interact in a way that allows them to share electrons and create a stable molecule.
Covalent bonding is a type of chemical bonding that involves the sharing of electron pairs between two atoms. This sharing of electrons occurs as a result of the overlapping of the atomic orbitals of the bonding atoms.
In a covalent bond, each atom contributes one or more electrons to the shared pair, and both atoms share the pair of electrons. The strength of the bond is determined by the extent of the overlap between the atomic orbitals involved.
The extent of the overlap is influenced by a number of factors, including the size and shape of the orbitals, the orientation of the orbitals relative to each other, and the energy of the orbitals. When the overlap is strong, the resulting covalent bond is also strong, and the molecule is more stable.
What is Required Orbital overlap and Covalent bond
For a covalent bond to form, a certain degree of orbital overlap is required between the atomic orbitals of the bonding atoms. The extent of overlap required for a covalent bond to form depends on several factors, such as the size, shape, and energy of the orbitals involved.
In general, for two atomic orbitals to overlap and form a covalent bond, they should have similar energy levels and orientation. The orbitals should also be of similar size and shape, with the same or similar electron densities in the region of overlap.
The degree of overlap between the orbitals determines the strength of the covalent bond. The greater the degree of overlap, the stronger the bond. This is because a greater overlap means that there is a stronger attraction between the nuclei and the shared electrons, which results in a more stable molecule.
In summary, the required orbital overlap for a covalent bond to form depends on several factors, including the size, shape, and energy of the orbitals involved, and the degree of overlap determines the strength of the resulting bond.
When is Required Orbital overlap and Covalent bond
“Required Orbital overlap” and “Covalent bond” are concepts that apply whenever two or more atoms come together to form a molecule through the sharing of electrons. Covalent bonds are one of the most common types of chemical bonds found in nature and are essential for the formation of many important compounds, including organic molecules like proteins, DNA, and carbohydrates.
Covalent bonds are formed when atomic orbitals of two atoms overlap and the electrons in these orbitals are shared between the two atoms. The degree of orbital overlap and the strength of the resulting covalent bond depend on several factors, including the size, shape, and energy of the orbitals involved.
Thus, these concepts apply whenever chemical bonds are formed through the sharing of electrons, which is a fundamental process in the field of chemistry.
Where is Required Orbital overlap and Covalent bond
“Required Orbital overlap” and “Covalent bond” are concepts in chemistry that apply in any situation where atoms come together to form a molecule through the sharing of electrons. This can occur in a variety of environments, including in the gas phase, in solution, or in solid-state materials.
For example, covalent bonds are essential for the formation of organic molecules like proteins and DNA in living organisms, as well as for the formation of many other important chemical compounds found in nature. Covalent bonds also play a crucial role in the formation of solid-state materials, such as crystals and polymers.
Thus, these concepts are applicable wherever chemical bonding occurs, which is a fundamental process in the natural world and is essential to many chemical and biological processes.
How is Required Orbital overlap and Covalent bond
“Required Orbital overlap” and “Covalent bond” are related concepts that describe the sharing of electrons between two or more atoms to form a chemical bond. The process of forming a covalent bond involves the overlapping of atomic orbitals of the bonding atoms.
The degree of overlap between the orbitals is determined by several factors, including the size, shape, and energy of the orbitals involved. When the orbitals overlap, the electrons in the overlapping region are shared between the two atoms, resulting in the formation of a covalent bond.
The strength of the covalent bond is determined by the degree of orbital overlap between the two atoms. The greater the overlap between the orbitals, the stronger the bond will be, and the more stable the resulting molecule will be.
The formation of a covalent bond through orbital overlap is a fundamental process in chemistry and plays a crucial role in the formation of many important compounds found in nature, including organic molecules like proteins and DNA.
Case Study on Orbital overlap and Covalent bond
One example of the importance of orbital overlap and covalent bonding is the formation of the carbon-carbon double bond in ethene (C2H4), which is an important organic molecule.
In ethene, each carbon atom contributes two valence electrons to form a total of four valence electrons available for bonding. These electrons are distributed among the three 2p orbitals and one 2s orbital of each carbon atom.
When two ethene molecules come together, the 2p orbitals of each carbon atom overlap to form a pi bond, while the 2s orbitals overlap to form a sigma bond. The pi bond is formed by the sideways overlap of the two 2p orbitals, which creates a region of electron density above and below the plane of the molecule. The sigma bond is formed by the end-to-end overlap of the two 2s orbitals, which creates a region of electron density along the axis of the bond.
The degree of orbital overlap between the carbon atoms determines the strength of the double bond in ethene. The pi bond is weaker than the sigma bond because the sideways overlap of the 2p orbitals is less efficient than the end-to-end overlap of the 2s orbitals. However, the combined strength of the two bonds is sufficient to hold the two carbon atoms together and form a stable molecule.
The formation of the carbon-carbon double bond in ethene is an example of the importance of orbital overlap and covalent bonding in organic chemistry. Without the ability of the 2p and 2s orbitals of the carbon atoms to overlap and form covalent bonds, the formation of organic molecules like ethene would not be possible.
White paper on Orbital overlap and Covalent bond
Introduction
Chemical bonding is a fundamental process in chemistry that is responsible for the formation of many important compounds found in nature. Two of the most common types of chemical bonding are ionic bonding and covalent bonding. In covalent bonding, two or more atoms share electrons to form a molecule. The process of covalent bonding involves orbital overlap, which is the overlap of atomic orbitals of the bonding atoms.
Orbital Overlap
Atomic orbitals are regions in space around the nucleus of an atom where electrons are most likely to be found. When two or more atoms come together to form a molecule, the atomic orbitals of the bonding atoms can overlap. The degree of overlap between the orbitals is determined by several factors, including the size, shape, and energy of the orbitals involved.
The overlap of orbitals results in the sharing of electrons between the two atoms. The shared electrons are held in the overlapping region by the attractive forces of both nuclei, resulting in the formation of a covalent bond. The degree of orbital overlap between the atoms determines the strength of the covalent bond. The greater the overlap between the orbitals, the stronger the bond will be, and the more stable the resulting molecule will be.
Covalent Bonding
Covalent bonding is essential for the formation of many important compounds found in nature, including organic molecules like proteins and DNA. Covalent bonding can occur between atoms of the same element or between atoms of different elements. When atoms of different elements bond covalently, the resulting compound is called a covalent compound.
In a covalent compound, the atoms are held together by the shared electrons between them. The electrons are shared in a way that creates a stable molecule with a low potential energy. The strength of the covalent bond is determined by the degree of overlap between the orbitals of the bonding atoms, as well as by the size, shape, and energy of the orbitals.
Conclusion
Orbital overlap and covalent bonding are fundamental concepts in chemistry that describe the sharing of electrons between two or more atoms to form a chemical bond. The process of orbital overlap and covalent bonding is essential for the formation of many important compounds found in nature, including organic molecules like proteins and DNA. The strength of the covalent bond is determined by the degree of orbital overlap between the atoms. The greater the overlap between the orbitals, the stronger the bond will be, and the more stable the resulting molecule will be.
Career Opportunities of Orbital overlap and Covalent bond
The study of orbital overlap and covalent bonding is an important area of chemistry with many career opportunities available to those with expertise in this field. Here are some of the career options for those interested in orbital overlap and covalent bonding:
- Organic chemist – Organic chemists study the properties and reactions of carbon-based molecules, which are held together by covalent bonds. They work in a wide range of industries, including pharmaceuticals, food, and cosmetics.
- Materials scientist – Materials scientists study the properties of materials and how they are formed. They often work on developing new materials with specific properties, such as strength, durability, and conductivity, which are held together by covalent bonds.
- Polymer chemist – Polymer chemists study the properties and reactions of large molecules made up of repeating units, which are held together by covalent bonds. They often work in the development of new materials for use in industries such as textiles, plastics, and adhesives.
- Chemical engineer – Chemical engineers work to design and develop chemical processes and equipment. They often work in industries such as manufacturing, energy, and pharmaceuticals, where covalent bonding plays a critical role in the development and production of products.
- Analytical chemist – Analytical chemists develop and use methods to analyze the chemical properties of substances. They often work in industries such as environmental testing, food and drug testing, and materials testing, where covalent bonding plays a critical role in determining the properties of substances.
- Research scientist – Research scientists work in a wide range of industries and academic settings, conducting research on a variety of topics related to chemistry, including orbital overlap and covalent bonding.
These are just a few examples of the many career opportunities available to those with expertise in orbital overlap and covalent bonding. A strong background in chemistry, along with specialized training in these areas, can lead to a rewarding career in research, industry, or academia.
