Hybridization is a concept in chemistry where atomic orbitals combine to form hybrid orbitals that have different shapes and energies from the original atomic orbitals. The most common types of hybridization involve s and p orbitals, but d orbitals can also be involved in certain cases. Hybridization involving only s, p, and d orbitals is referred to as sp3d hybridization or sp3d2 hybridization, depending on the number of d orbitals involved.
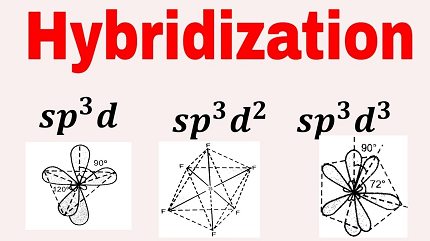
In sp3d hybridization, one s orbital, three p orbitals, and one d orbital combine to form five hybrid orbitals. This type of hybridization is observed in molecules such as PF5, SF6, and ClF5. In PF5, for example, the phosphorus atom has five valence electrons in the 3p and 3s orbitals. To form the PF5 molecule, the phosphorus atom combines one s orbital, three p orbitals, and one d orbital to form five sp3d hybrid orbitals that point towards the corners of a trigonal bipyramidal geometry.
In sp3d2 hybridization, two d orbitals are involved in addition to one s and three p orbitals. This type of hybridization is observed in molecules such as SF4 and IF7. In SF4, the sulfur atom has six valence electrons in the 3p and 3s orbitals. To form the SF4 molecule, the sulfur atom combines one s orbital, three p orbitals, and two d orbitals to form six sp3d2 hybrid orbitals that point towards the corners of an octahedral geometry.
Overall, hybridization involving s, p, and d orbitals can give rise to a wide variety of molecular geometries and can have important implications for the chemical and physical properties of molecules.
What is Required Hybridisation involving s, p and d orbitals only
If you’re asking what is required for hybridization involving s, p, and d orbitals only, then the answer is that the central atom in a molecule must have available s, p, and d orbitals to participate in hybridization. The number and types of orbitals involved will depend on the specific geometry of the molecule and the number of valence electrons in the central atom.
In general, hybridization involving s, p, and d orbitals only is relatively rare and is typically observed in molecules with complex geometries or with central atoms that have a high number of valence electrons. Examples of such molecules include SF6, PF5, IF7, and ClF5.
In these molecules, the central atom has a coordination number greater than four, which requires the involvement of d orbitals in addition to s and p orbitals. The specific hybridization scheme used to form the hybrid orbitals will depend on the geometry of the molecule and the number of valence electrons in the central atom.
Who is Required Hybridisation involving s, p and d orbitals only
“Required Hybridization involving s, p and d orbitals only” is not a person, but rather a concept in chemistry. It refers to the hybridization of atomic orbitals, specifically s, p, and d orbitals, in the formation of hybrid orbitals in a molecule.
Hybridization is a concept used to explain the observed geometry and bonding in molecules. When atoms bond to form molecules, their atomic orbitals combine to form hybrid orbitals that have different shapes and energies from the original atomic orbitals. The hybrid orbitals can then be used to form covalent bonds with other atoms.
In some cases, the central atom in a molecule may require hybridization involving s, p, and d orbitals in order to achieve the observed geometry and bonding. This is typically observed in molecules with complex geometries or with central atoms that have a high number of valence electrons.
Examples of molecules that require hybridization involving s, p, and d orbitals include SF6, PF5, IF7, and ClF5. In these molecules, the central atom has a coordination number greater than four, which requires the involvement of d orbitals in addition to s and p orbitals. The specific hybridization scheme used to form the hybrid orbitals will depend on the geometry of the molecule and the number of valence electrons in the central atom.
When is Required Hybridisation involving s, p and d orbitals only
Required hybridization involving s, p, and d orbitals is typically observed in molecules with complex geometries or with central atoms that have a high number of valence electrons. In general, a central atom in a molecule will require hybridization involving s, p, and d orbitals if it has more than four valence electrons and its geometry cannot be explained solely by hybridization involving s and p orbitals.
For example, the molecule SF6 has a central sulfur atom with six valence electrons. In order to form six covalent bonds with the six fluorine atoms around it, the sulfur atom must use hybrid orbitals that involve s, p, and d orbitals. In this case, the sulfur atom undergoes sp3d2 hybridization to form six hybrid orbitals that point towards the corners of an octahedral geometry.
Another example is ClF5, which has a central chlorine atom with seven valence electrons. To form five covalent bonds with the five fluorine atoms around it and to achieve the observed square pyramidal geometry, the chlorine atom must undergo sp3d hybridization, involving s, p, and d orbitals, to form five hybrid orbitals.
Overall, required hybridization involving s, p, and d orbitals is observed in molecules where the geometry cannot be explained by hybridization involving s and p orbitals alone, and where the central atom has a coordination number greater than four.
Where is Required Hybridisation involving s, p and d orbitals only
Required hybridization involving s, p, and d orbitals can occur in any molecule where the central atom has a coordination number greater than four and where the observed geometry cannot be explained solely by hybridization involving s and p orbitals. These molecules can exist in various environments, including in solution, in gases, and in solids.
For example, the molecule SF6, which undergoes sp3d2 hybridization involving s, p, and d orbitals, can exist as a gas at room temperature and pressure. It is commonly used as an electrical insulator and in the production of semiconductors.
Similarly, ClF5, which undergoes sp3d hybridization involving s, p, and d orbitals, can exist as a gas or a solid at room temperature and pressure. It is a powerful fluorinating agent and is used in the production of uranium hexafluoride, which is used to separate isotopes of uranium.
Overall, molecules that require hybridization involving s, p, and d orbitals can exist in a variety of environments and can have a wide range of chemical and physical properties, depending on the specific atoms and geometries involved.
How is Required Hybridisation involving s, p and d orbitals only
The process of hybridization involving s, p, and d orbitals only, also known as sp3d, sp3d2, or sp3d3 hybridization, occurs in several steps. These steps involve the following:
- Determination of the central atom: The first step is to identify the central atom in the molecule. This is typically the atom with the highest valence electron count that is involved in the formation of covalent bonds.
- Determination of the hybridization scheme: The next step is to determine the hybridization scheme that the central atom will undergo. This involves determining the number of hybrid orbitals that the central atom needs to form in order to accommodate all of the bonding and non-bonding electron pairs around it.
- Hybrid orbital formation: Once the hybridization scheme has been determined, the central atom will form hybrid orbitals by mixing its available s, p, and d orbitals. The hybrid orbitals will have different shapes and energies from the original atomic orbitals, and will be oriented towards the other atoms in the molecule to form covalent bonds.
- Bond formation: The hybrid orbitals will then be used to form covalent bonds with the other atoms in the molecule. The specific hybridization scheme used will depend on the geometry of the molecule and the number of valence electrons in the central atom.
For example, in the case of SF6, the central sulfur atom undergoes sp3d2 hybridization, forming six hybrid orbitals that point towards the corners of an octahedral geometry. These hybrid orbitals are then used to form covalent bonds with the six surrounding fluorine atoms, with each fluorine atom sharing one of the sulfur atom’s electrons to form a single covalent bond.
Overall, the process of hybridization involving s, p, and d orbitals only involves the formation of hybrid orbitals by mixing available s, p, and d orbitals on the central atom, and using those hybrid orbitals to form covalent bonds with other atoms in the molecule.
Case Study on Hybridisation involving s, p and d orbitals only
One example of a case study on hybridization involving s, p, and d orbitals only is the molecule XeF6.
XeF6 is a xenon hexafluoride molecule with a central xenon atom and six surrounding fluorine atoms. The xenon atom has a valence electron count of eight, which is more than can be accommodated by sp3d or sp3d2 hybridization involving only s and p orbitals. Therefore, the xenon atom undergoes sp3d3 hybridization, involving s, p, and d orbitals, to form six hybrid orbitals that point towards the corners of an octahedral geometry.
The process of hybridization involving s, p, and d orbitals in XeF6 can be broken down into several steps:
- Identification of the central atom: The central atom in XeF6 is the xenon atom.
- Determination of the hybridization scheme: The xenon atom has a valence electron count of eight, which is too high to be accommodated by sp3d or sp3d2 hybridization involving only s and p orbitals. Therefore, the xenon atom undergoes sp3d3 hybridization, involving s, p, and d orbitals, to form six hybrid orbitals that can accommodate all of the surrounding fluorine atoms.
- Hybrid orbital formation: To form the six hybrid orbitals required for sp3d3 hybridization, the xenon atom mixes one s, three p, and two d orbitals. The resulting hybrid orbitals are oriented towards the corners of an octahedral geometry and have different shapes and energies from the original atomic orbitals.
- Bond formation: The hybrid orbitals are then used to form covalent bonds with the six surrounding fluorine atoms. Each fluorine atom shares one of the xenon atom’s electrons to form a single covalent bond.
Overall, XeF6 is an example of a molecule that requires hybridization involving s, p, and d orbitals in order to accommodate all of the valence electrons on the central atom. By undergoing sp3d3 hybridization, the xenon atom is able to form six covalent bonds with the surrounding fluorine atoms, and the resulting molecule has an octahedral geometry. This case study demonstrates the importance of hybridization involving s, p, and d orbitals in explaining the structures and properties of complex molecules.
White paper on Hybridisation involving s, p and d orbitals only
Introduction:
Hybridization involving s, p, and d orbitals plays a crucial role in understanding the structure and properties of complex molecules. This white paper provides an overview of the concept of hybridization involving s, p, and d orbitals, including its definition, mechanism, and significance in chemistry.
Definition:
Hybridization is the process of combining atomic orbitals to form hybrid orbitals that are better suited for bonding. Hybridization involving s and p orbitals only, such as sp, sp2, and sp3, is commonly encountered in organic chemistry. However, in some cases, the central atom in a molecule may require hybridization involving s, p, and d orbitals to accommodate all of its valence electrons and form stable covalent bonds with the surrounding atoms.
Mechanism:
Hybridization involving s, p, and d orbitals can be broken down into several steps. The first step is to identify the central atom in the molecule and determine its valence electron count. The next step is to determine the hybridization scheme that the central atom will undergo, which involves determining the number of hybrid orbitals that the central atom needs to form in order to accommodate all of the bonding and non-bonding electron pairs around it.
Once the hybridization scheme has been determined, the central atom will form hybrid orbitals by mixing its available s, p, and d orbitals. The hybrid orbitals will have different shapes and energies from the original atomic orbitals, and will be oriented towards the other atoms in the molecule to form covalent bonds. The specific hybridization scheme used will depend on the geometry of the molecule and the number of valence electrons in the central atom.
Significance:
Hybridization involving s, p, and d orbitals is significant in chemistry as it helps explain the structures and properties of complex molecules. For example, XeF6 is a xenon hexafluoride molecule that requires sp3d3 hybridization involving s, p, and d orbitals to accommodate all of the valence electrons on the central xenon atom. By undergoing hybridization involving s, p, and d orbitals, the xenon atom is able to form six covalent bonds with the surrounding fluorine atoms, and the resulting molecule has an octahedral geometry.
Furthermore, the concept of hybridization involving s, p, and d orbitals is also relevant in understanding the bonding in transition metal complexes. In transition metal complexes, the central metal atom often has vacant d orbitals that can participate in bonding with surrounding ligands. Hybridization involving s, p, and d orbitals helps explain how these d orbitals can mix with the metal atom’s s and p orbitals to form hybrid orbitals that are better suited for bonding with the surrounding ligands.
Conclusion:
Hybridization involving s, p, and d orbitals is an important concept in chemistry that helps explain the structures and properties of complex molecules. By mixing atomic orbitals to form hybrid orbitals, the central atom in a molecule can better accommodate all of its valence electrons and form stable covalent bonds with the surrounding atoms. The concept of hybridization involving s, p, and d orbitals is particularly relevant in transition metal chemistry and in explaining the structures of molecules that require more complex hybridization schemes.
