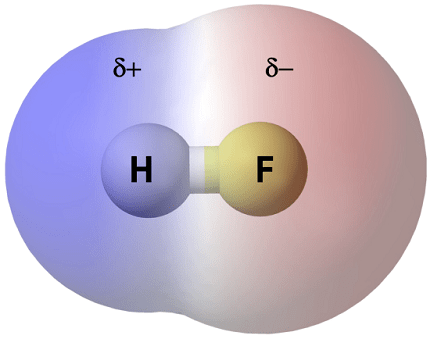
In chemistry, polarity refers to the separation of electric charge within a molecule or compound. A molecule can be polar or nonpolar depending on the electronegativity of its constituent atoms and the molecular geometry.
When two atoms with different electronegativities are bonded together, the electron pair in the bond is not shared equally, resulting in a dipole moment. The atom with the higher electronegativity attracts the shared electrons more strongly and acquires a partial negative charge, while the other atom becomes partially positive.
The polarity of a molecule depends not only on the polarity of its individual bonds but also on the molecular geometry. For example, a linear molecule like carbon dioxide (CO2) is nonpolar because the polar bonds between carbon and oxygen are oriented in opposite directions, canceling out the dipole moments. In contrast, a bent molecule like water (H2O) is polar because the polar bonds between hydrogen and oxygen are not oriented in opposite directions, resulting in a net dipole moment.
Polarity plays an important role in many chemical and physical properties of molecules, including solubility, boiling and melting points, and reactivity. For example, polar molecules tend to dissolve in polar solvents, while nonpolar molecules tend to dissolve in nonpolar solvents.
What is Required Polarity in molecules
The required polarity in a molecule depends on its intended use or function. In some cases, polarity may be necessary for the molecule to interact with other polar substances, such as in the case of water-soluble molecules. In other cases, nonpolarity may be necessary for a molecule to interact with nonpolar substances, such as in the case of oil-soluble molecules.
For example, in biological systems, polar molecules such as carbohydrates and proteins play important roles in cellular signaling, while nonpolar molecules such as lipids make up cell membranes. Similarly, in industrial applications, the polarity of a molecule may be important for its solubility in a particular solvent or for its ability to adhere to a surface.
In general, molecules with polar functional groups such as hydroxyl (-OH), amino (-NH2), or carboxyl (-COOH) tend to be polar, while molecules composed primarily of nonpolar carbon-carbon and carbon-hydrogen bonds tend to be nonpolar. However, the overall polarity of a molecule is also influenced by its three-dimensional shape and the distribution of its polar and nonpolar groups.
Nomenclature of Polarity in molecules
The nomenclature of polarity in molecules generally refers to the classification of molecules as polar or nonpolar based on their molecular structure and the distribution of electric charge within the molecule.
Polar molecules have a net dipole moment, meaning that there is an uneven distribution of electric charge within the molecule. They are typically characterized by the presence of polar covalent bonds, in which electrons are not shared equally between the atoms due to differences in their electronegativities. The polarity of a molecule is also influenced by its molecular geometry, as the arrangement of atoms in the molecule can affect the distribution of charge.
Nonpolar molecules, on the other hand, have a symmetrical distribution of electric charge within the molecule, with no net dipole moment. They are typically characterized by the absence of polar covalent bonds or by the presence of bonds in which the electronegativities of the atoms are similar.
The nomenclature of polarity in molecules is important in many areas of science and technology, including chemistry, biochemistry, materials science, and engineering. It is used to describe the physical and chemical properties of molecules, such as solubility, melting point, boiling point, and reactivity. Understanding the polarity of molecules is essential in many fields, as it can influence the behavior of molecules in chemical reactions and biological systems, as well as their applications in materials science and engineering.
When is Required Polarity in molecules
The required polarity in molecules is important in many different contexts, including in biology, chemistry, and materials science. In biological systems, the polarity of molecules can influence their interactions with other molecules and cellular components, as well as their transport across cell membranes. For example, polar molecules such as glucose and amino acids are transported across cell membranes by specific transporter proteins, while nonpolar molecules such as oxygen and carbon dioxide can diffuse through the lipid bilayer of the membrane.
In chemistry, the polarity of molecules can affect their reactivity and solubility in different solvents. Polar molecules tend to dissolve in polar solvents, while nonpolar molecules tend to dissolve in nonpolar solvents. This property is exploited in various chemical processes and separations, such as in chromatography.
In materials science, the polarity of molecules can influence the properties of materials such as surface energy, adhesion, and wetting behavior. For example, materials with high surface energy and polarity tend to adhere strongly to polar surfaces, while materials with low surface energy and nonpolarity tend to repel water and other polar substances.
Overall, the required polarity in molecules is important in many different fields and applications, and understanding and controlling this property is key to developing new materials, drugs, and other compounds with specific properties and functions.
Where is Required Polarity in molecules
The required polarity in molecules is a property of the molecule itself and is determined by the distribution of electric charge within the molecule. This distribution of charge is influenced by the electronegativity of the atoms that make up the molecule and the geometry of the molecule.
The electronegativity of an atom is its ability to attract electrons towards itself in a covalent bond. If two atoms with different electronegativities are bonded together, the shared electrons will be pulled towards the more electronegative atom, creating a dipole moment in the bond. The polarity of a molecule is determined by the sum of the dipole moments of its constituent bonds and the geometry of the molecule.
For example, in a water molecule (H2O), the oxygen atom is more electronegative than the hydrogen atoms, resulting in a polar covalent bond. The geometry of the molecule is bent, which means that the two O-H bonds are not opposite each other, resulting in a net dipole moment for the molecule.
In summary, the required polarity in molecules is a property of the molecule itself and is determined by the electronegativity of its constituent atoms and the geometry of the molecule.
How is Required Polarity in molecules
The required polarity in molecules is determined by the electronegativity of the atoms that make up the molecule and the geometry of the molecule. The electronegativity of an atom is a measure of its ability to attract electrons towards itself in a covalent bond.
If two atoms with different electronegativities are bonded together, the shared electrons will be pulled towards the more electronegative atom, creating a dipole moment in the bond. The polarity of a molecule is determined by the sum of the dipole moments of its constituent bonds and the geometry of the molecule.
For example, in a water molecule (H2O), the oxygen atom is more electronegative than the hydrogen atoms, resulting in a polar covalent bond. The geometry of the molecule is bent, which means that the two O-H bonds are not opposite each other, resulting in a net dipole moment for the molecule.
To determine the polarity of a molecule, one can calculate the vector sum of the individual bond dipole moments, taking into account the geometry of the molecule. If the vector sum is zero, the molecule is nonpolar, while if the vector sum is nonzero, the molecule is polar.
Overall, the required polarity in molecules is determined by the fundamental properties of the atoms and bonds that make up the molecule, as well as the three-dimensional geometry of the molecule itself.
Case Study on Polarity in molecules
A good case study on polarity in molecules is the comparison of two organic compounds: ethanol and ethane.
Ethanol (C2H5OH) is a polar molecule with a net dipole moment due to the presence of an -OH (hydroxyl) functional group. The oxygen atom in the hydroxyl group is more electronegative than the carbon and hydrogen atoms, which results in a partial negative charge on the oxygen and a partial positive charge on the hydrogen atoms. This partial separation of charges creates a dipole moment in the molecule, giving it polarity.
On the other hand, ethane (C2H6) is a nonpolar molecule made up of only carbon and hydrogen atoms that are connected by single covalent bonds. Because the electronegativities of carbon and hydrogen atoms are very similar, there is no significant difference in electronegativity between them. Therefore, the electron pair in each covalent bond is equally shared, resulting in no separation of charges and no dipole moment in the molecule.
The difference in polarity between ethanol and ethane has important implications for their properties and uses. For example, ethanol is water-soluble due to its polarity and ability to form hydrogen bonds with water molecules. This makes it a useful solvent for polar substances, and it is commonly used in the production of pharmaceuticals, cosmetics, and fuels. In contrast, ethane is nonpolar and is not soluble in water. Instead, it is used as a fuel for heating and cooking, as well as a feedstock for the production of other chemicals.
Overall, the comparison of ethanol and ethane illustrates the importance of polarity in determining the properties and uses of molecules in various contexts.
White paper on Polarity in molecules
Introduction:
Polarity is an important property of molecules that affects their physical and chemical properties. The polarity of a molecule is determined by the distribution of electric charge within the molecule, which in turn is influenced by the electronegativity of the atoms that make up the molecule and the geometry of the molecule. This white paper aims to provide an overview of the concept of polarity in molecules and its significance in various fields.
Polarity in Molecules:
A molecule is said to be polar if it has a net dipole moment, meaning that the distribution of electric charge within the molecule is not uniform. This dipole moment is created by the separation of positive and negative charges in the molecule due to differences in electronegativity between the atoms that make up the molecule. Electronegativity is a measure of an atom’s ability to attract electrons towards itself in a covalent bond. When two atoms with different electronegativities are bonded together, the shared electrons are pulled towards the more electronegative atom, creating a dipole moment in the bond.
The polarity of a molecule is determined by the sum of the dipole moments of its constituent bonds and the geometry of the molecule. For example, water (H2O) is a polar molecule because the oxygen atom is more electronegative than the hydrogen atoms, resulting in a polar covalent bond. The geometry of the molecule is bent, which means that the two O-H bonds are not opposite each other, resulting in a net dipole moment for the molecule.
In contrast, a molecule is said to be nonpolar if it has no net dipole moment. This occurs when the electronegativities of the atoms in the molecule are the same or similar, resulting in an equal sharing of electrons and no separation of charges. For example, ethane (C2H6) is a nonpolar molecule made up of only carbon and hydrogen atoms that are connected by single covalent bonds. Because the electronegativities of carbon and hydrogen atoms are very similar, there is no significant difference in electronegativity between them.
Significance of Polarity in Molecules:
The polarity of a molecule has important implications for its physical and chemical properties, as well as its uses in various fields. For example, polar molecules such as water are excellent solvents for other polar substances, as they can form hydrogen bonds with the solute molecules. This property makes water an essential component of biological systems, as it enables many biomolecules to dissolve and interact with each other.
In addition, the polarity of a molecule can affect its boiling point, melting point, and viscosity. Polar molecules tend to have higher boiling points and melting points than nonpolar molecules of similar size and shape, as they can form stronger intermolecular forces such as hydrogen bonds. This property is exploited in various industries such as petrochemicals, where the boiling points of different hydrocarbons are used to separate them by distillation.
Conclusion:
In summary, polarity is an important property of molecules that is determined by the electronegativity of the atoms that make up the molecule and the geometry of the molecule. The polarity of a molecule affects its physical and chemical properties, including its solubility, boiling point, melting point, and viscosity. Understanding the concept of polarity is essential in various fields such as chemistry, biology, materials science, and engineering, and can help us design and optimize new materials and molecules for specific applications.
