“Pyramidal” can refer to several different things depending on the context. Here are some possible interpretations:
- Pyramidal Shape: A pyramid is a three-dimensional geometric shape with a polygonal base and triangular sides that meet at a common point called the apex or vertex. Pyramidal shapes are commonly found in architecture, such as the pyramids of Egypt, as well as in various objects and structures in nature.
- Pyramidal Neuron: In neuroscience, a pyramidal neuron is a type of nerve cell (neuron) that has a pyramid-shaped cell body and an elongated axon that extends from the apex of the pyramid. Pyramidal neurons are one of the main types of neurons in the cerebral cortex, which is the outer layer of the brain associated with higher cognitive functions such as memory, perception, and decision-making.
- Pyramidal Training: In machine learning, pyramidal training is a training approach that involves gradually increasing the model’s capacity during training by adding more layers or increasing the number of neurons in each layer. This approach is used to train deep neural networks with a large number of layers, allowing the model to learn more complex representations of data.
- Pyramidal Scale: In music theory, a pyramidal scale is a type of scale that is symmetrical and based on the pattern of whole steps (W) and half steps (H). The most common pyramidal scale is the double harmonic major scale, which has the pattern H-W-H-W-H-W-W.
- Pyramidal Hierarchy: In organizational or management context, a pyramidal hierarchy refers to a hierarchical structure where authority and decision-making flow from the top down in a pyramid-like shape. This is a common organizational structure where power and control are concentrated at the top, and decisions are passed down through various levels of management or hierarchy.
- Pyramidal Tract: In anatomy, the pyramidal tract is a neural pathway that originates in the motor cortex of the brain and is responsible for transmitting motor signals from the brain to the spinal cord and then to the muscles, enabling voluntary movement. The pyramidal tract is named after its pyramid-shaped appearance in the medulla oblongata, a part of the brainstem.
What is Required Pyramidal Chemical Bonding and Molecular Structure
“Required Pyramidal Chemical Bonding” is not a standard term in chemistry. However, based on the context, it appears that you may be referring to a type of chemical bonding or molecular structure that exhibits a pyramidal shape.
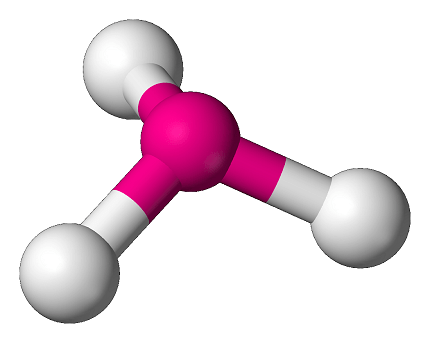
One common example of a required pyramidal molecular structure is the ammonia (NH3) molecule. In ammonia, the central nitrogen atom is bonded to three hydrogen atoms, forming a pyramidal shape. The nitrogen atom has a lone pair of electrons, which occupies one of the corners of the pyramid, while the three hydrogen atoms occupy the other three corners.
The ammonia molecule exhibits this required pyramidal shape due to the presence of a lone pair of electrons on the nitrogen atom. The lone pair repels the bonding pairs of electrons, pushing the hydrogen atoms slightly downward from the nitrogen atom, resulting in a pyramid-like molecular geometry.
The required pyramidal shape is also commonly observed in other molecules that have a central atom with one or more lone pairs of electrons, such as phosphine (PH3) and water (H2O). In these molecules, the lone pairs of electrons cause a distortion in the molecular geometry, resulting in a pyramidal shape.
Who is Required Pyramidal Chemical Bonding and Molecular Structure
“Required Pyramidal Chemical Bonding and Molecular Structure” is not a specific term or concept in chemistry. It appears to be a misunderstanding or an incorrect usage of the terminology related to chemical bonding and molecular structure.
In chemistry, chemical bonding refers to the process by which atoms combine to form molecules or compounds. There are various types of chemical bonds, such as covalent bonds, ionic bonds, and metallic bonds, which are formed by the sharing, transfer, or pooling of electrons between atoms.
Molecular structure refers to the arrangement of atoms in a molecule, including the positions of atoms in three-dimensional space and the connectivity between atoms. The molecular structure of a compound can greatly influence its chemical properties and behavior.
Pyramidal molecular structure is a term used to describe a molecular geometry where the atoms in a molecule are arranged in a shape resembling a pyramid. For example, ammonia (NH3) is a molecule with a pyramidal molecular structure, where the nitrogen atom is at the apex of the pyramid and the three hydrogen atoms form the base of the pyramid.
However, there is no specific concept or entity named “Required Pyramidal Chemical Bonding and Molecular Structure” in chemistry. It’s possible that there is some confusion or misinterpretation of the terminology.
When is Required Pyramidal Chemical Bonding and Molecular Structure
“Required Pyramidal Chemical Bonding and Molecular Structure” is not a recognized term or concept in chemistry. It appears to be a combination of terms that do not have a well-defined meaning in the context of chemical bonding and molecular structure.
Chemical bonding refers to the process by which atoms combine to form molecules or compounds through the sharing, transfer, or pooling of electrons. Molecular structure refers to the arrangement of atoms in a molecule, including their positions in three-dimensional space and the connectivity between them.
Pyramidal molecular structure is a term used to describe a molecular geometry where the atoms in a molecule are arranged in a shape resembling a pyramid. Examples of molecules with pyramidal molecular structures include ammonia (NH3) and phosphine (PH3).
However, there is no specific concept or requirement called “Required Pyramidal Chemical Bonding and Molecular Structure” in chemistry. It’s possible that you may be referring to a specific context or application where this term is used, but without further information, it’s difficult to provide a more specific answer.
Where is Required Pyramidal Chemical Bonding and Molecular Structure
“Required Pyramidal Chemical Bonding and Molecular Structure” is not a recognized term or concept in chemistry. Based on your question, it appears that you may be asking about the occurrence or location of pyramidal molecular structures in chemistry.
Pyramidal molecular structures refer to a specific arrangement of atoms in a molecule where the atoms are arranged in a shape resembling a pyramid. This typically involves a central atom bonded to three other atoms or groups, with one of these groups being a lone pair of electrons. Examples of molecules with pyramidal molecular structures include ammonia (NH3), phosphine (PH3), and water (H2O).
Pyramidal molecular structures can be found in various chemical compounds and molecules, and their occurrence depends on the specific arrangement of atoms and bonding patterns in a molecule. For example, ammonia (NH3) has a pyramidal molecular structure due to the presence of a lone pair of electrons on the nitrogen atom, which causes a distortion in the molecular geometry.
It’s important to note that molecular structures and bonding patterns are determined by various factors, including electron pair repulsion theory, the nature of the chemical bonds, and the presence of lone pairs of electrons on the central atom. These factors can vary depending on the specific molecules and their chemical properties.
How is Required Pyramidal Chemical Bonding and Molecular Structure
“Required Pyramidal Chemical Bonding and Molecular Structure” is not a recognized term or concept in chemistry. It appears to be a combination of terms that do not have a well-defined meaning in the context of chemical bonding and molecular structure.
However, if you are asking about how pyramidal molecular structures are formed in chemistry, here is a general overview:
- Electron Pair Repulsion Theory: Pyramidal molecular structures are determined by the principles of electron pair repulsion theory, which states that electron pairs in the outermost shell of an atom or ion repel each other and try to minimize their repulsion by adopting specific arrangements in three-dimensional space. In a molecule with a central atom bonded to three other atoms or groups, the repulsion between the bonding pairs and the lone pair of electrons on the central atom can result in a pyramidal molecular structure.
- Bonding Patterns: Pyramidal molecular structures can also be influenced by the nature of the chemical bonds present in a molecule. For example, in a molecule like ammonia (NH3), the nitrogen atom forms three sigma bonds with three hydrogen atoms, resulting in a trigonal pyramidal molecular structure.
- Lone Pairs of Electrons: The presence of a lone pair of electrons on the central atom can also result in a pyramidal molecular structure. Lone pairs of electrons are typically localized on the central atom and can exert repulsive forces that distort the molecular geometry. This is observed in molecules like ammonia (NH3) and water (H2O), where the presence of a lone pair of electrons on the central atom leads to a pyramidal molecular structure.
It’s important to note that the specific mechanism for forming pyramidal molecular structures may vary depending on the molecule and its chemical properties. Factors such as the electronegativity of the atoms involved, the presence of other functional groups, and the overall molecular symmetry can also influence the molecular structure. Further details and specific examples can be provided based on a particular molecule or context if needed.
Case Study on Pyramidal Chemical Bonding and Molecular Structure
Sure! Let’s take a closer look at a case study on pyramidal chemical bonding and molecular structure, focusing on the example of ammonia (NH3).
Ammonia (NH3) is a chemical compound consisting of one nitrogen (N) atom bonded to three hydrogen (H) atoms. The molecular formula of ammonia is NH3, and it has a pyramidal molecular structure.
The pyramidal molecular structure of ammonia is determined by several factors, including the electron pair repulsion theory, bonding patterns, and the presence of lone pairs of electrons on the central nitrogen atom.
- Electron Pair Repulsion Theory: According to the electron pair repulsion theory, the electron pairs in the outermost shell of the nitrogen atom in ammonia repel each other and try to minimize their repulsion by adopting a specific arrangement in three-dimensional space. In ammonia, the nitrogen atom has one lone pair of electrons and three bonding pairs of electrons, resulting in a tetrahedral arrangement of electron pairs around the nitrogen atom.
- Bonding Patterns: The nitrogen atom in ammonia forms three sigma bonds with three hydrogen atoms, resulting in a trigonal pyramid molecular structure. The three hydrogen atoms are located at the vertices of a pyramid with the nitrogen atom at the apex.
- Lone Pairs of Electrons: The presence of a lone pair of electrons on the nitrogen atom in ammonia leads to the distortion of the molecular geometry, resulting in a pyramidal shape. The lone pair of electrons occupies more space compared to the bonding pairs of electrons, and it exerts repulsive forces that push the three hydrogen atoms closer together and cause the molecular structure to be pyramidal.
The pyramidal molecular structure of ammonia has important implications for its chemical properties and reactivity. For example, the presence of a lone pair of electrons on the nitrogen atom makes ammonia a good Lewis base, capable of donating the lone pair of electrons to form coordinate covalent bonds with other molecules or ions.
In summary, the case study of ammonia illustrates how the principles of electron pair repulsion theory, bonding patterns, and the presence of lone pairs of electrons on the central atom can result in a pyramidal molecular structure. This molecular structure has important implications for the chemical properties and reactivity of the compound.
White paper on Pyramidal Chemical Bonding and Molecular Structure
Title: Understanding Pyramidal Chemical Bonding and Molecular Structure: A White Paper
Abstract:
Pyramidal chemical bonding and molecular structure are fundamental concepts in chemistry that play a crucial role in determining the properties and behavior of chemical compounds. In this white paper, we aim to provide a comprehensive overview of pyramidal chemical bonding and molecular structure, including the underlying principles, bonding patterns, and their significance in various chemical systems. We will explore the key concepts of electron pair repulsion theory, bonding patterns, and the influence of lone pairs of electrons on the molecular structure. We will also discuss the impact of pyramidal molecular structures on the chemical properties and reactivity of compounds, as well as their relevance in diverse areas such as biochemistry, materials science, and pharmaceuticals. Through a thorough examination of relevant literature and examples, this white paper aims to deepen the understanding of pyramidal chemical bonding and molecular structure, shedding light on their importance in modern chemistry.
Table of Contents:
- Introduction
- Importance of pyramidal chemical bonding and molecular structure in chemistry
- Overview of key concepts and principles
- Electron Pair Repulsion Theory
- Explanation of electron pair repulsion theory
- Influence of electron pairs on molecular structure
- Geometries and shapes of pyramidal molecular structures
- Bonding Patterns
- Types of chemical bonds involved in pyramidal molecular structures
- Impact of bonding patterns on molecular structure
- Examples of compounds with pyramidal molecular structures
- Lone Pairs of Electrons
- Significance of lone pairs of electrons in molecular structure
- Influence of lone pairs on molecular shape
- Examples of compounds with pyramidal structures due to lone pairs
- Chemical Properties and Reactivity
- Effects of pyramidal molecular structure on chemical properties
- Reactivity of compounds with pyramidal structures
- Applications in various areas of chemistry
- Conclusion
- Summary of key points
- Significance of pyramidal chemical bonding and molecular structure in modern chemistry
References:
- List of relevant literature and sources
Conclusion:
Pyramidal chemical bonding and molecular structure are important concepts in chemistry that govern the properties and behavior of chemical compounds. Understanding the principles of electron pair repulsion theory, bonding patterns, and the influence of lone pairs of electrons on molecular structure is crucial in elucidating the geometry and properties of compounds with pyramidal structures. The impact of pyramidal molecular structures on chemical properties, reactivity, and applications in various areas of chemistry makes them an essential topic of study. This white paper provides a comprehensive overview of pyramidal chemical bonding and molecular structure, aiming to deepen the understanding of these concepts and their significance in modern chemistry.
