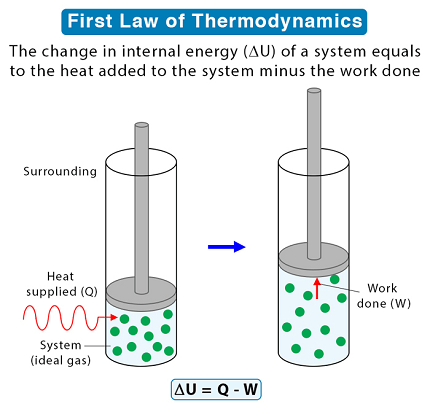
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another or transferred from one system to another. In other words, the total amount of energy in a closed system remains constant. This law is fundamental to understanding many physical and chemical processes, including the behavior of gases, the flow of heat, and the behavior of engines and other machines. The first law of thermodynamics can be expressed mathematically as:
ΔU = Q – W
Where ΔU is the change in internal energy of a system, Q is the heat added to the system, and W is the work done by the system. This equation shows that any energy added to a system must either be used to do work or to increase the internal energy of the system.
What is Required First law of thermodynamics
The first law of thermodynamics is required to understand the fundamental principles of energy conservation in physical and chemical systems. It provides a framework for understanding how energy is transformed and transferred between different forms and different systems. Without the first law of thermodynamics, it would not be possible to accurately predict or analyze the behavior of systems that involve the transfer or transformation of energy.
The first law of thermodynamics is essential in many fields, including physics, chemistry, engineering, and environmental science. For example, it is used to understand the behavior of heat engines, such as car engines and power plants, and to analyze the energy efficiency of these systems. It is also used to understand the behavior of chemical reactions and to determine the energy required or released in these reactions.
Overall, the first law of thermodynamics is a fundamental principle that is required for understanding energy conservation and the behavior of physical and chemical systems.
Who is Required First law of thermodynamics
The first law of thermodynamics is a fundamental principle of physics that was first proposed by a German physicist, Rudolf Clausius, in the mid-19th century. However, it was later refined and further developed by other scientists, including James Joule and Hermann von Helmholtz.
James Joule is known for his pioneering work on the relationship between heat and mechanical work, which helped establish the first law of thermodynamics. Joule’s experiments demonstrated that mechanical work could be converted into heat energy, and vice versa, with no net gain or loss of energy. This idea became a key component of the first law of thermodynamics.
Hermann von Helmholtz, a German physicist and physician, also made significant contributions to the development of the first law of thermodynamics. He is known for his work on the conservation of energy and his formulation of the principle of conservation of energy, which became a fundamental principle in physics.
Overall, the first law of thermodynamics is the result of the contributions of several scientists, including Clausius, Joule, and Helmholtz, among others.
When is Required First law of thermodynamics
The first law of thermodynamics is required whenever there is a need to understand the transfer or transformation of energy in physical or chemical systems. It is applicable to a wide range of systems and processes, including:
- Heat engines: The first law of thermodynamics is essential for understanding the behavior of heat engines, which convert thermal energy into mechanical work. It is used to analyze the efficiency of these systems and to determine the amount of work that can be extracted from a given amount of heat.
- Chemical reactions: The first law of thermodynamics is also important for understanding the energy changes that occur in chemical reactions. It is used to calculate the heat released or absorbed during a reaction and to predict whether a reaction will be exothermic or endothermic.
- Phase transitions: The first law of thermodynamics is also applicable to phase transitions, such as melting or vaporization. It is used to calculate the amount of energy required to change the phase of a substance.
- Environmental science: The first law of thermodynamics is important in environmental science, where it is used to understand energy flows in ecosystems and to analyze the impact of human activities on the environment.
Overall, the first law of thermodynamics is required whenever there is a need to understand the transfer or transformation of energy in physical or chemical systems. It is a fundamental principle that is applicable to a wide range of fields and applications.
Where is Required First law of thermodynamics
The first law of thermodynamics is required in a wide range of physical and chemical systems, and is applicable in many fields, including physics, chemistry, engineering, and environmental science. It is a fundamental principle that governs the behavior of energy in all closed systems, and is used to understand the transfer and transformation of energy in these systems.
Here are a few examples of where the first law of thermodynamics is required:
- Heat engines: The first law of thermodynamics is essential for understanding the behavior of heat engines, such as car engines and power plants. These systems convert thermal energy into mechanical work, and the first law is used to analyze the efficiency of these systems and to determine the amount of work that can be extracted from a given amount of heat.
- Chemical reactions: The first law of thermodynamics is also important for understanding the energy changes that occur in chemical reactions. It is used to calculate the heat released or absorbed during a reaction and to predict whether a reaction will be exothermic or endothermic.
- Environmental science: The first law of thermodynamics is important in environmental science, where it is used to understand energy flows in ecosystems and to analyze the impact of human activities on the environment.
- Geology: The first law of thermodynamics is used to understand the behavior of heat and energy in geological processes, such as the formation of rocks and minerals, and the transfer of heat within the Earth’s crust.
Overall, the first law of thermodynamics is required in a wide range of physical and chemical systems, and is applicable in many fields and applications.
How is Required First law of thermodynamics
The first law of thermodynamics is a fundamental principle that states that energy cannot be created or destroyed, only transferred or transformed from one form to another. This law is based on the conservation of energy, which means that the total energy in a closed system remains constant.
The first law of thermodynamics can be expressed mathematically as follows:
ΔU = Q – W
where ΔU is the change in internal energy of the system, Q is the heat transferred into the system, and W is the work done by the system.
This equation shows that the change in internal energy of a system is equal to the heat transferred into the system minus the work done by the system. In other words, the energy that enters a system must either be used to do work or increase the internal energy of the system.
The first law of thermodynamics is used to understand the transfer and transformation of energy in physical and chemical systems. For example, it is used to analyze the efficiency of heat engines, to calculate the energy changes that occur in chemical reactions, and to understand energy flows in ecosystems.
Overall, the first law of thermodynamics is a fundamental principle that provides a framework for understanding the behavior of energy in all closed systems. It is essential in many fields and applications, and is used to predict and analyze the transfer and transformation of energy in a wide range of physical and chemical systems.
Case Study on First law of thermodynamics
One example of the application of the first law of thermodynamics is in the analysis of a simple heat engine. A heat engine is a device that converts heat energy into mechanical work, and is a key component of many industrial processes and technologies.
Consider a simple heat engine that consists of a cylinder containing a gas, a piston that can move up and down in the cylinder, and a heat source and sink. The heat source is a hot reservoir, while the heat sink is a cold reservoir. The piston is connected to a crankshaft that can be used to perform mechanical work.
When the heat engine is started, heat is transferred from the hot reservoir to the gas in the cylinder, causing it to expand and push the piston up. As the piston moves up, the gas does work on the crankshaft, which is used to perform mechanical work. After the gas has expanded, it is allowed to cool and contract, which causes the piston to move back down and complete the cycle.
The first law of thermodynamics can be used to analyze the behavior of this heat engine. The law states that the change in internal energy of the system (ΔU) is equal to the heat transferred into the system (Q) minus the work done by the system (W):
ΔU = Q – W
In the case of the heat engine, the heat transferred into the system is the heat received from the hot reservoir, while the work done by the system is the work performed by the piston on the crankshaft. Thus, we can write:
ΔU = Q_hot – W
where Q_hot is the heat transferred from the hot reservoir to the gas in the cylinder.
The efficiency of the heat engine can be defined as the ratio of the work done by the engine to the heat transferred into the engine. Using the first law of thermodynamics, we can write:
η = W / Q_hot
where η is the efficiency of the engine.
From this equation, we can see that the efficiency of the engine depends on the amount of work that can be extracted from the heat transferred into the system. This in turn depends on the temperature difference between the hot and cold reservoirs.
Overall, this case study illustrates how the first law of thermodynamics can be used to analyze the behavior of a simple heat engine. The law provides a framework for understanding the transfer and transformation of energy in physical systems, and is essential for the design and optimization of many industrial processes and technologies.
White paper on First law of thermodynamics
Introduction:
The first law of thermodynamics is a fundamental principle that states that energy cannot be created or destroyed, only transferred or transformed from one form to another. This principle is based on the conservation of energy, which means that the total energy in a closed system remains constant. The first law of thermodynamics is a cornerstone of thermodynamics and is used to understand the transfer and transformation of energy in physical and chemical systems. In this white paper, we will discuss the first law of thermodynamics in detail, including its history, significance, and applications.
History:
The concept of the conservation of energy can be traced back to the work of several scientists in the 19th century, including Julius Robert Mayer, James Joule, and Hermann von Helmholtz. These scientists conducted experiments that demonstrated the relationship between heat, work, and energy, and laid the groundwork for the first law of thermodynamics.
In 1850, Rudolf Clausius formulated the first law of thermodynamics as part of his work on the theory of heat. He stated that “The energy of the universe is constant; the first law of thermodynamics.” This statement encapsulated the idea that energy cannot be created or destroyed, only transformed from one form to another.
Significance:
The first law of thermodynamics is significant because it provides a framework for understanding the behavior of energy in closed systems. This principle is essential in many fields and applications, including physics, chemistry, engineering, and biology. The first law of thermodynamics is used to predict and analyze the transfer and transformation of energy in a wide range of physical and chemical systems, including heat engines, chemical reactions, and ecosystems.
Applications:
Heat engines: The first law of thermodynamics is used to analyze the behavior of heat engines, which are devices that convert heat energy into mechanical work. This law is used to understand the efficiency of heat engines and to optimize their design and performance.
Chemical reactions: The first law of thermodynamics is used to understand the energy changes that occur in chemical reactions. This law is used to predict whether a chemical reaction will be exothermic (releasing heat) or endothermic (absorbing heat).
Ecosystems: The first law of thermodynamics is used to understand energy flows in ecosystems. This law is used to predict the amount of energy available to organisms in an ecosystem and to understand how energy is transferred from one trophic level to another.
Conclusion:
The first law of thermodynamics is a fundamental principle that states that energy cannot be created or destroyed, only transferred or transformed from one form to another. This principle is based on the conservation of energy, which means that the total energy in a closed system remains constant. The first law of thermodynamics is significant because it provides a framework for understanding the behavior of energy in closed systems. This principle is used in many fields and applications, including physics, chemistry, engineering, and biology. Understanding the first law of thermodynamics is essential for predicting and analyzing the transfer and transformation of energy in a wide range of physical and chemical systems.
