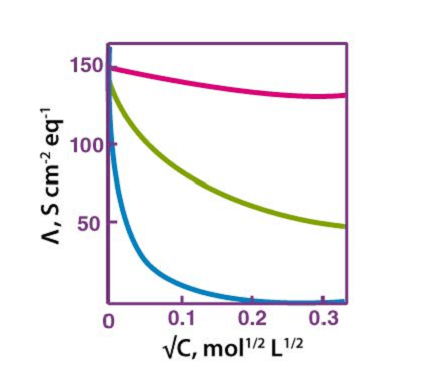
Kohlrausch’s law, also known as Kohlrausch’s displacement law, states that the molar conductivity of an electrolyte can be expressed as the sum of the contributions of its individual ions.
In other words, the total conductivity of an electrolyte solution is equal to the sum of the conductivities of the cations and anions present in the solution. This law holds true for dilute solutions of strong electrolytes at infinite dilution, where the ions are assumed to be completely dissociated.
The law is often written as: λ_m = λ_+ + λ_-
where λ_m is the molar conductivity of the electrolyte, λ_+ is the molar conductivity of the cation, and λ_- is the molar conductivity of the anion.
Kohlrausch’s law is important in the field of electrochemistry because it allows for the determination of the molar conductivity of an electrolyte by measuring the conductivity of its individual ions. This can be useful in understanding the behavior of electrolytes in solution and in the design of electrochemical devices.
What is Required Kohlrausch’s law
To apply Kohlrausch’s law, the following conditions must be met:
- The electrolyte should be a strong electrolyte: The law is applicable only for strong electrolytes, which are substances that completely dissociate into ions when dissolved in water.
- The electrolyte should be in a dilute solution: The law holds true only for solutions where the concentration of the electrolyte is low enough to assume that the ions are completely dissociated.
- The temperature of the solution should be constant: The law is valid only at a constant temperature because the molar conductivity of an electrolyte varies with temperature.
- The electrodes should be non-polarizable: Non-polarizable electrodes are required to measure the conductivity of the individual ions without interference from the electrodes.
By satisfying these conditions, Kohlrausch’s law can be used to determine the molar conductivity of an electrolyte and provide insights into its behavior in solution.
Who is Required Kohlrausch’s law
Kohlrausch’s law is a scientific principle in the field of electrochemistry named after the German physicist Friedrich Kohlrausch. He developed this law in the late 19th century while studying the behavior of electrolytes in solution.
Friedrich Kohlrausch was born on October 14, 1840, in Rinteln, Germany, and died on January 17, 1910, in Marburg, Germany. He was a professor of physics at the University of Strasbourg and later at the University of Würzburg, where he conducted his seminal work on electrolyte conductivity.
Kohlrausch’s law has significant implications in electrochemistry and has led to further research on the behavior of electrolytes in solution. It is widely used today to study the properties of electrolyte solutions and in the design of electrochemical devices such as batteries and fuel cells.
When is Required Kohlrausch’s law
Kohlrausch’s law is used in various situations where the molar conductivity of an electrolyte needs to be determined. Some examples of when Kohlrausch’s law is required include:
- Determining the degree of dissociation of a strong electrolyte: By measuring the molar conductivity of an electrolyte and its individual ions, Kohlrausch’s law can be used to calculate the degree of dissociation of a strong electrolyte.
- Estimating the equivalent conductivity of an electrolyte at infinite dilution: Kohlrausch’s law can be used to estimate the equivalent conductivity of an electrolyte at infinite dilution, which is the maximum conductivity that an electrolyte can attain.
- Designing electrochemical devices: Kohlrausch’s law is used to design electrochemical devices such as batteries and fuel cells, where the behavior of electrolytes in solution is critical to the performance of the device.
- Studying the properties of electrolyte solutions: The law is also used to study the properties of electrolyte solutions and their behavior under different conditions such as temperature and concentration.
In general, Kohlrausch’s law is used whenever there is a need to determine the molar conductivity of an electrolyte and understand its behavior in solution.
Where is Required Kohlrausch’s law
Kohlrausch’s law is used in various fields where the behavior of electrolytes in solution is critical. Some common applications of Kohlrausch’s law include:
- Analytical chemistry: The law is used in analytical chemistry to determine the molar conductivity of electrolytes and understand their properties in solution. This information is essential for the analysis of various chemical compounds and their behavior under different conditions.
- Electrochemistry: Kohlrausch’s law plays a crucial role in electrochemistry, where the design and performance of electrochemical devices such as batteries and fuel cells rely on the behavior of electrolytes in solution.
- Chemical engineering: The law is used in chemical engineering to design and optimize industrial processes involving electrolyte solutions, such as the production of chemicals and pharmaceuticals.
- Environmental science: Kohlrausch’s law is also used in environmental science to study the behavior of electrolytes in natural water systems, such as rivers and lakes, and their impact on the environment.
Overall, Kohlrausch’s law finds applications in various fields where the properties of electrolyte solutions need to be understood and manipulated.
How is Required Kohlrausch’s law
Kohlrausch’s law is applied by measuring the molar conductivity of an electrolyte solution and its individual ions. The following steps are typically involved:
- Preparation of the electrolyte solution: A dilute solution of the electrolyte is prepared, and its concentration is determined. It is essential to ensure that the solution is sufficiently dilute to assume complete dissociation of the ions.
- Measurement of the molar conductivity of the electrolyte: The molar conductivity of the electrolyte solution is measured using a conductivity meter. The conductivity of the solution is then divided by its concentration to obtain the molar conductivity.
- Measurement of the conductivity of the individual ions: The conductivity of the individual ions present in the electrolyte is measured by preparing a solution containing only cations or anions and measuring their conductivities separately.
- Calculation of the molar conductivity of the individual ions: The molar conductivity of the cation and anion is calculated using the measured conductivity values and the solution’s concentration.
- Application of Kohlrausch’s law: The molar conductivity of the electrolyte solution is calculated using Kohlrausch’s law by summing the molar conductivities of the cation and anion.
By following these steps, Kohlrausch’s law can be used to determine the molar conductivity of an electrolyte and provide insights into its behavior in solution.
Case Study on Kohlrausch’s law
Here is a case study on the application of Kohlrausch’s law in the analysis of a strong electrolyte solution:
A chemist wants to determine the degree of dissociation of a strong electrolyte, sodium chloride (NaCl), in a 0.1 M solution at room temperature. The molar conductivity of the NaCl solution was measured using a conductivity meter and found to be 126.3 S cm^2 mol^-1. The conductivity of the individual ions, Na+ and Cl-, were measured separately and found to be 50.1 S cm^2 mol^-1 and 76.2 S cm^2 mol^-1, respectively.
The chemist can use Kohlrausch’s law to determine the degree of dissociation of NaCl by calculating the molar conductivity of the individual ions and comparing it with the measured molar conductivity of the electrolyte solution.
The molar conductivity of the cation, Na+, can be calculated as follows:
Molar conductivity of Na+ = Conductivity of Na+ x (1000 / concentration of solution) = 50.1 S cm^2 mol^-1 x (1000 / 0.1 M) = 501 S cm^2 mol^-1
Similarly, the molar conductivity of the anion, Cl-, can be calculated as follows:
Molar conductivity of Cl- = Conductivity of Cl- x (1000 / concentration of solution) = 76.2 S cm^2 mol^-1 x (1000 / 0.1 M) = 762 S cm^2 mol^-1
According to Kohlrausch’s law, the molar conductivity of NaCl can be calculated as the sum of the molar conductivities of the individual ions:
Molar conductivity of NaCl = Molar conductivity of Na+ + Molar conductivity of Cl- = 501 S cm^2 mol^-1 + 762 S cm^2 mol^-1 = 1263 S cm^2 mol^-1
The degree of dissociation of NaCl can be calculated using the formula:
Degree of dissociation = (Molar conductivity of NaCl – Molar conductivity of Na+) / Molar conductivity of NaCl
Substituting the values, we get:
Degree of dissociation = (1263 S cm^2 mol^-1 – 501 S cm^2 mol^-1) / 1263 S cm^2 mol^-1 = 0.603 or 60.3%
Therefore, the degree of dissociation of NaCl in the 0.1 M solution is 60.3%, indicating that a significant fraction of NaCl molecules dissociate into Na+ and Cl- ions in solution.
This case study illustrates the use of Kohlrausch’s law in the analysis of a strong electrolyte solution, where the molar conductivity of the electrolyte and its individual ions are measured to determine the degree of dissociation of the electrolyte.
White paper on Kohlrausch’s law
Introduction
Kohlrausch’s law is a fundamental concept in the field of physical chemistry that relates the molar conductivity of an electrolyte solution to the molar conductivities of its constituent ions. It was formulated by the German physicist Friedrich Kohlrausch in 1875 and has since found extensive use in the analysis of electrolyte solutions. This white paper provides an overview of Kohlrausch’s law, its applications, and limitations.
Kohlrausch’s Law
Kohlrausch’s law states that the molar conductivity (Λ) of an electrolyte solution is the sum of the molar conductivities of its constituent ions. Mathematically, it can be expressed as:
Λ_m = Σ_± λ_± C_±
Where Λ_m is the molar conductivity of the electrolyte solution, λ_± is the molar conductivity of the cation (+) or anion (-), and C_± is the concentration of the cation or anion in the solution.
Kohlrausch’s law assumes that the electrolyte is completely dissociated in solution, i.e., all of the ions are free to move and carry charge. This assumption is valid for strong electrolytes, such as NaCl, that undergo complete dissociation in solution. For weak electrolytes, such as acetic acid, the degree of dissociation is low, and Kohlrausch’s law cannot be applied directly.
Applications of Kohlrausch’s Law
Kohlrausch’s law has several applications in the analysis of electrolyte solutions, some of which are discussed below:
- Determination of degree of dissociation: Kohlrausch’s law can be used to determine the degree of dissociation of a strong electrolyte in solution. The molar conductivity of the electrolyte solution and its constituent ions are measured, and the degree of dissociation is calculated using the law’s mathematical expression.
- Prediction of transport properties: The molar conductivity of an electrolyte solution provides information about the ions’ mobility and their interactions with the solvent. This information can be used to predict the solution’s transport properties, such as its diffusion coefficient and ionic conductivity.
- Analysis of complex electrolytes: Electrolyte solutions often contain multiple ions that interact with each other, leading to deviations from ideal behavior. Kohlrausch’s law can be used to analyze such complex electrolytes by measuring the molar conductivities of the individual ions and using them to predict the behavior of the electrolyte as a whole.
Limitations of Kohlrausch’s Law
While Kohlrausch’s law is a useful tool for analyzing electrolyte solutions, it has several limitations, some of which are discussed below:
- Assumption of complete dissociation: Kohlrausch’s law assumes that the electrolyte is completely dissociated in solution, which is only valid for strong electrolytes. For weak electrolytes, the degree of dissociation is low, and the law cannot be applied directly.
- Influence of solvent: The properties of the solvent, such as its viscosity and dielectric constant, can affect the mobility of the ions in solution and, consequently, the molar conductivity of the electrolyte. Kohlrausch’s law does not account for such solvent effects.
- Limitations in the low-concentration regime: Kohlrausch’s law is valid only in the low-concentration regime, where the ions’ interactions are negligible. At higher concentrations, the ions’ interactions can lead to deviations from ideal behavior, and the law’s validity may be limited.
Conclusion
Kohlrausch’s law is an essential concept in the field of physical chemistry that relates the molar conductivity of an electrolyte solution to the molar conductivities of its constituent ions. It has several applications in the analysis of electrolyte solutions, including the determination of the degree of dissociation, prediction of transport properties, and analysis of complex electrolytes. However, the law’s validity is limited by its assumption of complete dissociation, its inability to account for solvent effects, and its validity in the low-concentration regime only. Overall, Kohlrausch’s law provides a valuable tool for understanding the behavior of electrolyte solutions and their constituent ions.
