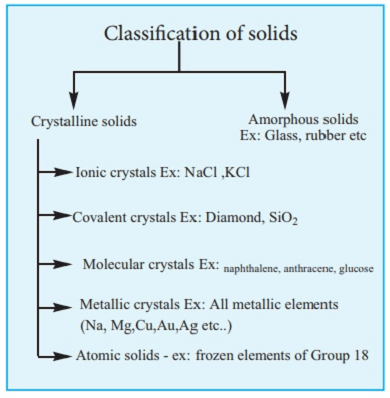
Solids can be classified into various types based on different criteria. Here are some common ways of classifying solids:
- Based on atomic/molecular arrangement:
a. Crystalline solids: These are solids in which the atoms/molecules are arranged in a regular and repeating pattern. Examples include diamonds, salt, and metals.
b. Amorphous solids: These are solids in which the atoms/molecules are arranged in a random manner, without any long-range order. Examples include glass, rubber, and some plastics.
- Based on electrical conductivity:
a. Conductors: These are solids that allow electricity to flow through them easily. Examples include metals and some metal alloys.
b. Insulators: These are solids that do not allow electricity to flow through them easily. Examples include glass, rubber, and some plastics.
c. Semiconductors: These are solids that have electrical conductivity between conductors and insulators. Examples include silicon, germanium, and some metal oxides.
- Based on the type of intermolecular forces:
a. Ionic solids: These are solids in which the atoms/molecules are held together by ionic bonds. Examples include salt, MgO, and CaCl2.
b. Covalent solids: These are solids in which the atoms/molecules are held together by covalent bonds. Examples include diamond, SiO2, and some polymers.
c. Metallic solids: These are solids in which the atoms/molecules are held together by metallic bonds. Examples include copper, iron, and gold.
d. Molecular solids: These are solids in which the atoms/molecules are held together by weak intermolecular forces. Examples include ice, sugar, and some organic compounds.
- Based on the degree of order/disorder:
a. Single crystals: These are solids in which the entire crystal is made up of a single continuous piece of material, with a regular and repeating pattern of atoms/molecules.
b. Polycrystalline solids: These are solids in which the crystal is made up of many smaller crystals, with different orientations and patterns.
c. Glasses: These are amorphous solids that have been cooled from a liquid state too quickly to allow the atoms/molecules to arrange themselves in a regular pattern.
These are some of the common ways of classifying solids. However, some solids can fall into multiple categories depending on the criteria used for classification.
What is Required Classification of solids
The required classification of solids depends on the specific application or field of study. For example, a materials scientist may classify solids based on their mechanical properties or thermal conductivity, while a chemist may classify solids based on their chemical properties and reactions.
In general, a useful classification of solids should help to differentiate between different types of solids based on their properties, behavior, and structure. It should also provide insights into the underlying physics, chemistry, or materials science principles that govern their behavior.
A comprehensive classification of solids would take into account all relevant factors, such as their atomic/molecular arrangement, electrical conductivity, intermolecular forces, degree of order/disorder, mechanical properties, thermal properties, and chemical properties. However, depending on the specific application or field of study, some factors may be more important than others.
In summary, the required classification of solids depends on the purpose for which it is being used, and should take into account all relevant factors that help to differentiate between different types of solids based on their properties and behavior.
Who is Required Classification of solids
The classification of solids is required by a wide range of professionals and researchers who work with solid materials, including:
- Materials scientists and engineers who study the properties and behavior of solids for various applications, such as electronic devices, construction materials, and medical implants.
- Chemists who study the chemical properties and reactions of solids, such as catalysts, drugs, and pigments.
- Physicists who study the underlying physical principles that govern the behavior of solids, such as crystal structure, thermal conductivity, and magnetic properties.
- Geologists who study the properties and behavior of natural solids, such as minerals and rocks.
- Environmental scientists and engineers who study the fate and transport of contaminants in solid media, such as soils and sediments.
- Architects and builders who use different types of solids for construction materials, such as bricks, concrete, and steel.
- Forensic scientists who use solid materials to analyze evidence and solve crimes, such as fingerprints and DNA.
In summary, the classification of solids is required by a diverse range of professionals and researchers who work with solid materials in different applications and fields of study.
When is Required Classification of solids
The classification of solids is required at various stages of research and development, manufacturing, and application of solid materials. Some specific instances when the classification of solids may be required include:
- Research and development of new materials: When developing new materials for various applications, researchers need to classify the materials based on their properties, behavior, and structure to understand their potential uses and limitations.
- Materials selection for manufacturing: When selecting materials for manufacturing products, manufacturers need to classify the materials based on their properties and behavior to ensure that they are suitable for the intended application.
- Quality control during manufacturing: During the manufacturing process, quality control personnel need to classify the materials based on their properties and behavior to ensure that the final product meets the required specifications.
- Analysis of solid samples: In scientific investigations and forensic analysis, solid materials are often analyzed to determine their composition, structure, and behavior. Classification of solids is required to identify and differentiate between different types of solid materials.
- Environmental monitoring: In environmental monitoring, solid materials such as soil and sediment samples are collected and classified based on their properties and behavior to determine the presence and concentration of contaminants.
In summary, the classification of solids is required at various stages of research, development, manufacturing, and application of solid materials in different industries and fields of study.
Where is Required Classification of solids
The classification of solids is required in various locations where solid materials are used, processed, or analyzed. Some specific locations where the classification of solids may be required include:
- Laboratories: Laboratories are common locations where researchers and scientists analyze solid materials to determine their properties and behavior. The classification of solids is required to differentiate between different types of materials and to understand their characteristics.
- Manufacturing facilities: Manufacturing facilities use solid materials to produce various products. The classification of solids is required to ensure that the materials used in manufacturing are suitable for the intended application.
- Construction sites: Solid materials such as bricks, concrete, and steel are commonly used in construction. The classification of solids is required to ensure that the materials used are appropriate for the specific application and meet the required standards.
- Environmental monitoring sites: Environmental monitoring sites collect soil and sediment samples to analyze the presence and concentration of contaminants. The classification of solids is required to identify and differentiate between different types of solids present in the samples.
- Forensic laboratories: Forensic laboratories analyze solid materials such as fingerprints, DNA, and other evidence to solve crimes. The classification of solids is required to identify and differentiate between different types of materials present in the evidence.
In summary, the classification of solids is required in various locations where solid materials are used, processed, or analyzed in different industries and fields of study.
How is Required Classification of solids
The classification of solids can be achieved through various methods and techniques, depending on the specific properties and behavior of the solid materials being studied. Some common methods for the classification of solids include:
- Crystal structure analysis: Crystal structure analysis is used to determine the atomic or molecular arrangement of solid materials. This method is commonly used to classify solids such as metals, ceramics, and minerals.
- Mechanical testing: Mechanical testing is used to determine the mechanical properties of solid materials, such as strength, stiffness, and toughness. This method is commonly used to classify solids such as metals, polymers, and composites.
- Thermal analysis: Thermal analysis is used to determine the thermal properties of solid materials, such as thermal conductivity and thermal expansion. This method is commonly used to classify solids such as ceramics and polymers.
- Chemical analysis: Chemical analysis is used to determine the chemical properties of solid materials, such as their reactivity and composition. This method is commonly used to classify solids such as minerals, catalysts, and pharmaceuticals.
- Electrical and magnetic testing: Electrical and magnetic testing is used to determine the electrical and magnetic properties of solid materials. This method is commonly used to classify solids such as semiconductors and magnetic materials.
In summary, the classification of solids can be achieved through various methods and techniques, depending on the specific properties and behavior of the solid materials being studied. The choice of method depends on the specific application or field of study and the properties of the materials being classified.
Case Study on Classification of solids
Case Study: Classification of Ceramic Materials
Ceramic materials are a diverse group of solids that are widely used in various applications, such as electronics, aerospace, biomedical, and energy. The classification of ceramic materials is important for understanding their properties, behavior, and potential applications. In this case study, we will discuss the classification of ceramic materials based on their crystal structure.
Crystal structure analysis is a common method for the classification of ceramic materials. Ceramic materials can be classified into two main categories based on their crystal structure: crystalline ceramics and amorphous ceramics.
- Crystalline ceramics: Crystalline ceramics have a well-defined crystal structure and are composed of atoms or ions arranged in a specific pattern. Crystalline ceramics can be further classified into different types based on the crystal structure. Some common types of crystalline ceramics include:a. Ionic ceramics: Ionic ceramics are composed of positively and negatively charged ions arranged in a crystal lattice. Examples of ionic ceramics include alumina, zirconia, and calcium phosphate.b. Covalent ceramics: Covalent ceramics are composed of covalent bonds between atoms and have a high melting point and hardness. Examples of covalent ceramics include silicon carbide, boron nitride, and diamond.c. Molecular ceramics: Molecular ceramics are composed of molecules held together by weak intermolecular forces. Examples of molecular ceramics include zeolites, clays, and silica gel.
- Amorphous ceramics: Amorphous ceramics do not have a well-defined crystal structure and are composed of disordered atomic arrangements. Amorphous ceramics can be further classified into different types based on their structure and properties. Some common types of amorphous ceramics include:a. Glass ceramics: Glass ceramics are produced by controlled crystallization of glass and have both glassy and crystalline properties. Examples of glass ceramics include lithium disilicate and leucite-reinforced glass ceramics.b. Non-oxide ceramics: Non-oxide ceramics are composed of non-metallic elements and have high melting points and chemical resistance. Examples of non-oxide ceramics include carbides, nitrides, and borides.c. Composite ceramics: Composite ceramics are composed of two or more ceramic materials and have tailored properties. Examples of composite ceramics include ceramic-matrix composites and ceramic-polymer composites.
In summary, ceramic materials can be classified based on their crystal structure into crystalline ceramics and amorphous ceramics. Crystalline ceramics can be further classified based on the crystal structure into ionic ceramics, covalent ceramics, and molecular ceramics. Amorphous ceramics can be further classified based on their structure and properties into glass ceramics, non-oxide ceramics, and composite ceramics. The classification of ceramic materials is important for understanding their properties and potential applications in different industries and fields of study.
White paper on Classification of solids
Introduction
Classification of solids is an important aspect of materials science and engineering. It is the process of categorizing solids based on their physical and chemical properties, structures, and behavior. The classification of solids is essential for understanding their characteristics, applications, and potential uses in various fields. This white paper will provide an overview of the classification of solids, including its importance, methods, and examples.
Importance of Classification of Solids
The classification of solids is essential for several reasons. First, it helps to identify the unique properties of a solid, which is crucial for understanding its behavior and potential applications. For example, metals have excellent electrical conductivity, while ceramics have high strength and resistance to heat. Understanding these properties helps to determine the suitability of a material for a particular application.
Second, classification of solids is important for designing new materials with specific properties. By knowing the properties of different materials, it is possible to create composite materials with unique properties tailored for specific applications. For example, adding carbon fibers to a polymer matrix creates a composite material with high strength and stiffness.
Methods of Classification of Solids
There are several methods for classifying solids. These include crystal structure analysis, mechanical testing, thermal analysis, chemical analysis, electrical and magnetic testing, and optical microscopy. Each method focuses on different aspects of the solid, such as structure, properties, and behavior.
Crystal structure analysis is a widely used method for classifying solids. It involves the determination of the arrangement of atoms or ions in a crystal lattice. Based on the crystal structure, solids can be classified as metals, ceramics, minerals, or polymers.
Mechanical testing involves measuring the mechanical properties of solids, such as strength, stiffness, and toughness. This method is commonly used to classify solids such as metals, polymers, and composites.
Thermal analysis involves measuring the thermal properties of solids, such as thermal conductivity and thermal expansion. This method is commonly used to classify solids such as ceramics and polymers.
Chemical analysis involves determining the chemical properties of solids, such as reactivity and composition. This method is commonly used to classify solids such as minerals, catalysts, and pharmaceuticals.
Electrical and magnetic testing involves measuring the electrical and magnetic properties of solids. This method is commonly used to classify solids such as semiconductors and magnetic materials.
Optical microscopy involves analyzing the microstructure of solids using a microscope. This method is commonly used to classify solids such as metals and ceramics.
Examples of Classification of Solids
One example of the classification of solids is the classification of ceramics. Ceramics can be classified based on their crystal structure into crystalline ceramics and amorphous ceramics. Crystalline ceramics can be further classified into different types based on the crystal structure, such as ionic ceramics, covalent ceramics, and molecular ceramics. Amorphous ceramics can be further classified into different types based on their structure and properties, such as glass ceramics, non-oxide ceramics, and composite ceramics.
Another example of the classification of solids is the classification of polymers. Polymers can be classified based on their structure into linear polymers, branched polymers, and cross-linked polymers. They can also be classified based on their properties, such as thermoplastics, thermosets, and elastomers.
Conclusion
Classification of solids is an essential aspect of materials science and engineering. It involves categorizing solids based on their physical and chemical properties, structures, and behavior. The classification of solids is important for understanding their unique characteristics and potential applications. There are several methods for classifying solids, including crystal structure analysis, mechanical testing, thermal analysis, chemical analysis, electrical and magnetic testing, and optical microscopy. By understanding the classification of solids, it is possible to design new materials with tailored properties for specific applications.
