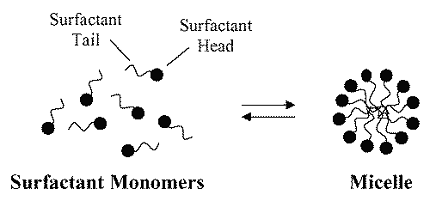
Surfactants are compounds that reduce the surface tension between two different substances, typically between a liquid and a gas or between two immiscible liquids. The word “surfactant” is a contraction of “surface active agent.” Surfactants have a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail, which allows them to interact with both water and oil. Some common examples of surfactants include soaps, detergents, and emulsifiers.
Micelles, on the other hand, are aggregates of surfactant molecules that form in a liquid when the concentration of surfactant reaches a certain threshold, called the critical micelle concentration (CMC). Micelles have a spherical shape, with the hydrophobic tails of the surfactant molecules facing inward and the hydrophilic heads facing outward. This arrangement allows the micelles to solubilize hydrophobic substances in the surrounding liquid, making them more stable and easier to handle. Some common examples of micelles include those formed by soap molecules in water, which help to emulsify and remove dirt and oil from surfaces.
Surfactant
Surfactants are substance intensifies that decline the surface pressure or interfacial strain between two fluids, a fluid and a gas, or a fluid and a strong. Surfactants might work as emulsifiers, wetting specialists, cleansers, frothing specialists, or dispersants. “Surfactant” is a mix of surface-dynamic specialist, begat c. 1950.
Specialists that increment surface pressure are “surface dynamic” in the exacting sense yet are not called surfactants as their impact is inverse to the normal significance. A typical illustration of surface pressure increment is salting out: adding an inorganic salt to a fluid arrangement of a pitifully polar substance will make the substance encourage. The substance may itself be a surfactant – this is one reason why numerous surfactants are ineffectual in ocean water.
Micelle
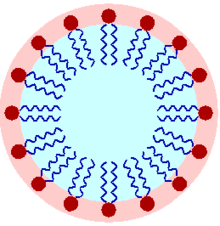
A micelle (/maɪˈsɛl/) or micella (/maɪˈsɛlə/) (plural micelles or micellae, separately) is a total (or supramolecular gathering) of surfactant amphipathic lipid particles scattered in a fluid, shaping a colloidal suspension (otherwise called related colloidal framework). A commonplace micelle in water frames a total with the hydrophilic “head” locales in touch with encompassing dissolvable, sequestering the hydrophobic single-tail districts in the micelle place.
This stage is brought about by the pressing way of behaving of single-tail lipids in a bilayer. The trouble filling all the volume of the inside of a bilayer, while obliging the region per head bunch constrained on the particle by the hydration of the lipid head bunch, prompts the development of the micelle. This kind of micelle is known as a typical stage micelle (oil-in-water micelle). Opposite micelles have the head bunches at the middle with the tails stretching out (water-in-oil micelle).
Micelles are roughly circular in shape. Different stages, including shapes like ellipsoids, chambers, and bilayers, are additionally conceivable. The shape and size of a micelle are a component of the sub-atomic calculation of its surfactant particles and arrangement conditions like surfactant fixation, temperature, pH, and ionic strength. The most common way of shaping micelles is known as micellisation and structures part of the stage conduct of numerous lipids as indicated by their polymorphism.
Block copolymer micelles
The idea of micelles was acquainted with portray the center crown totals of little surfactant particles, but it has likewise reached out to depict totals of amphiphilic block copolymers in specific solvents. Knowing the distinction between these two systems is significant. The significant contrast between these two sorts of totals is in the size of their structure blocks. Surfactant particles have a sub-atomic weight which is for the most part of two or three many grams for every mole while block copolymers are for the most part a couple of significant degrees bigger. Besides, because of the bigger hydrophilic and hydrophobic parts, block copolymers can have a significantly more articulated amphiphilic nature when contrasted with surfactant particles.
Due to these distinctions in the structure impedes, some block copolymer micelles act like surfactant ones, while others don’t. It is important thusly to make a qualification between the two circumstances. The previous ones will have a place with the powerful micelles while the last option will be called dynamically frozen micelles.
Dynamic micelles
Certain amphiphilic block copolymer micelles show a comparative way of behaving as surfactant micelles. These are by and large called dynamic micelles and are portrayed by a similar unwinding processes relegated to surfactant trade and micelle scission/recombination. Albeit the unwinding processes are similar between the two kinds of micelles, the energy of unimer trade are altogether different. While in surfactant frameworks the unimers leave and join the micelles through a dispersion controlled process, for copolymers the passage rate steady is more slow than a dissemination controlled process. The pace of this interaction was viewed as a diminishing power-law of the level of polymerization of the hydrophobic block to the power 2/3. This distinction is because of the winding of the hydrophobic block of a copolymer leaving the center of a micelle.
Block copolymers which structure dynamic micelles are a portion of the tri-block poloxamers under the right circumstances.
Kinetically frozen micelles
At the point when block copolymer micelles don’t show the trademark unwinding cycles of surfactant micelles, these are called actively frozen micelles. These can be accomplished in two ways: when the unimers shaping the micelles are not dissolvable in that frame of mind of the micelle arrangement, or on the other hand assuming the center shaping blocks are lustrous at the temperature wherein the micelles are found. Dynamically frozen micelles are shaped when both of these circumstances is met. An exceptional model where both of these circumstances are legitimate is that of polystyrene-b-poly(ethylene oxide). This block copolymer is portrayed by the high hydrophobicity of the center framing block, PS, which makes the unimers be insoluble in water. Besides, PS has a high glass change temperature which is, contingent upon the sub-atomic weight, higher than room temperature. On account of these two qualities, a water arrangement of PS-PEO micelles of adequately high sub-atomic weight can be considered dynamically frozen. This implies that none of the unwinding processes, which would drive the micelle arrangement towards thermodynamic balance, are conceivable. Spearheading work on these micelles was finished by Adi Eisenberg. It was likewise shown how the absence of unwinding processes permitted extraordinary opportunity in the potential morphologies framed. Also, the strength against weakening and tremendous scope of morphologies of actively frozen micelles make them especially fascinating, for instance, for the advancement of long flowing medication conveyance nanoparticles.
Surfactants in paint
Paint has four significant parts: shades, folios, solvents, and added substances. Shades give paint its tone, surface, strength, as well as deciding whether a paint is hazy or not. Normal white shades incorporate titanium dioxide and zinc oxide. Covers are the film framing part of a paint as it dries and influences the toughness, gleam, and adaptability of the covering. Polyurethanes, polyesters, and acrylics are instances of normal covers. The dissolvable is the medium wherein any remaining parts of the paint are disintegrated and vanishes away as the paint dries and fixes. The dissolvable likewise alters the relieving rate and thickness of the paint in its fluid state. There are two sorts of paint: dissolvable borne and water-borne paints. Dissolvable borne paints utilize natural solvents as the essential vehicle conveying the strong parts in a paint definition, though water-borne paints use water as the ceaseless medium. The added substances that are integrated into paints are a large number of things which give significant impacts on the properties of the paint and the last covering. Normal paint added substances are impetuses, thickeners, stabilizers, emulsifiers, texturizers, biocides to battle bacterial development, and so forth.
Fundamental article: Surfactant
The word surfactant is short for surface dynamic specialist. Surfactants are intensifies that bring down the surface strain of a fluid, the interfacial pressure between two fluids, or the interfacial pressure between a fluid and a strong. In arrangements this conduct is known as wetting, and it happens because of surfactants adsorbing to the air/water interface. Solvent surfactants are likewise equipped for shaping micelles and other total designs in arrangement, prompting a balancing out impact in plastic paints. Surfactants in paint are utilized to change many end properties of a dried paint, as well as to emulsify paints in their fluid state.
Problems with surfactant use
Environmental issues
Surfactants can weaken poisonous natural mixtures in paint which can enter the climate and make adverse consequences. Water-solvent surfactants can clean out of dried paints and enter the climate. A portion of these surfactants are straightforwardly harmful to creatures and the climate as well as increment the capacity of other poisonous pollutants present to enter the climate.
Cost
The expense of surfactants is somewhat reliant upon the raw petroleum market. As a stock element for creation of surfactants, paints exceptionally reliant upon surfactants will be impacted by this market. More complex surfactants with bigger, more challenging to integrate structure are more costly to create and have a more prominent outcome on end market cost of their applications. Thus, basic, simple to create and all the more harmless to the ecosystem surfactants are utilized all the more generally.
Case Study on Surfactants and micelles (only definitions and examples)
Sure, here is a case study on surfactants and micelles:
Case study: Surfactants and Micelles in Cleaning Products
A company that produces cleaning products is looking to improve the effectiveness of their products while also reducing their environmental impact. They decide to explore the use of surfactants and micelles in their products.
Surfactants are compounds that reduce surface tension between two substances, typically between a liquid and a gas or between two immiscible liquids. They have a hydrophilic (water-loving) head and a hydrophobic (water-repelling) tail. This allows them to interact with both water and oil. In cleaning products, surfactants are used to break up and emulsify oils and other hydrophobic substances so that they can be easily rinsed away with water.
Micelles are aggregates of surfactant molecules that form in a liquid when the concentration of surfactant reaches a certain threshold, called the critical micelle concentration (CMC). Micelles have a spherical shape, with the hydrophobic tails of the surfactant molecules facing inward and the hydrophilic heads facing outward. This arrangement allows the micelles to solubilize hydrophobic substances in the surrounding liquid, making them more stable and easier to handle.
The cleaning product company decides to incorporate surfactants and micelles into their products. They choose a variety of surfactants, including anionic, cationic, and nonionic surfactants, to improve the emulsification of oils and other hydrophobic substances. They also use micelles to solubilize and remove dirt and other particles from surfaces.
The company conducts a series of tests to compare the effectiveness of their new surfactant and micelle-based cleaning products to their existing products. They find that the new products are more effective at removing dirt and oils from surfaces, and that they require less water to rinse away the cleaning solution, reducing the environmental impact of the products.
In conclusion, the use of surfactants and micelles can improve the effectiveness of cleaning products while also reducing their environmental impact. By choosing a variety of surfactants and incorporating micelles into their products, the cleaning product company was able to create a more effective and sustainable product line.
White paper on Surfactants and micelles (only definitions and examples)
Here is a white paper on surfactants and micelles:
Introduction:
Surfactants and micelles are important compounds that have numerous applications in various fields, including chemistry, biology, and engineering. Surfactants are molecules that have both hydrophilic and hydrophobic regions, which enable them to interact with both water and non-polar compounds. Micelles, on the other hand, are aggregates of surfactant molecules that form in solution at a certain concentration, known as the critical micelle concentration (CMC). This paper will explore the definitions and examples of surfactants and micelles.
Surfactants:
Surfactants are compounds that reduce the surface tension between two different substances, typically between a liquid and a gas or between two immiscible liquids. Surfactants have a hydrophilic head and a hydrophobic tail, which allows them to interact with both water and oil. The hydrophilic head is typically charged or polar, while the hydrophobic tail is usually an alkyl or aryl group. Surfactants can be classified into four types: anionic, cationic, nonionic, and amphoteric.
Anionic surfactants are negatively charged and are commonly used in cleaning products such as laundry detergents, dishwashing liquids, and shampoos. Examples of anionic surfactants include sodium lauryl sulfate, sodium laureth sulfate, and sodium dodecylbenzenesulfonate.
Cationic surfactants are positively charged and are used as fabric softeners, hair conditioners, and disinfectants. Examples of cationic surfactants include benzalkonium chloride, cetrimonium chloride, and cetylpyridinium chloride.
Nonionic surfactants are uncharged and are commonly used in personal care products, household cleaners, and industrial applications. Examples of nonionic surfactants include polyethylene glycol, ethoxylated alcohols, and sorbitan esters.
Amphoteric surfactants have both positive and negative charges and are used in personal care products and detergents. Examples of amphoteric surfactants include cocamidopropyl betaine, lauryl betaine, and disodium cocoamphodiacetate.
Micelles:
Micelles are aggregates of surfactant molecules that form in a liquid when the concentration of surfactant reaches a certain threshold, called the critical micelle concentration (CMC). Micelles have a spherical shape, with the hydrophobic tails of the surfactant molecules facing inward and the hydrophilic heads facing outward. This arrangement allows the micelles to solubilize hydrophobic substances in the surrounding liquid, making them more stable and easier to handle. The size of micelles depends on the structure and concentration of the surfactant molecules.
Micelles have numerous applications in various fields. In chemistry, micelles can be used to solubilize and stabilize hydrophobic compounds, to separate and purify proteins, and to deliver drugs. In biology, micelles are used as models for cell membranes and as vehicles for drug delivery. In engineering, micelles are used in emulsion polymerization and in the production of nanomaterials.
Conclusion:
In conclusion, surfactants and micelles are important compounds with a wide range of applications in various fields. Surfactants have both hydrophilic and hydrophobic regions that allow them to interact with both water and non-polar compounds, and they can be classified into four types: anionic, cationic, nonionic, and amphoteric. Micelles are aggregates of surfactant molecules that form in a liquid at a certain concentration, known as the critical micelle concentration (CMC). Micelles have a spherical shape with the hydrophobic tails of the surfactant molecules facing inward and the hydrophilic heads facing outward. They have numerous applications in chemistry, biology, and engineering, including drug delivery, emulsion polymerization, and the production of nanomaterials. By understanding the properties and applications of surfactants and micelles, scientists and engineers can develop new and innovative technologies to solve complex problems in various industries.
