
Hydroxides are chemical compounds that consist of a metal cation and a hydroxide anion (OH-). They are a type of base that reacts with acids to form water and a salt. Hydroxides are important in many industrial and chemical processes, such as the production of soaps and detergents, water treatment, and the extraction of metals from ores.
Some common examples of hydroxides include sodium hydroxide (NaOH), also known as caustic soda, potassium hydroxide (KOH), calcium hydroxide (Ca(OH)2), also known as slaked lime, and magnesium hydroxide (Mg(OH)2), also known as milk of magnesia. These hydroxides have various applications in industries such as food and beverage, agriculture, and medicine.
Hydroxides can also have important environmental implications, particularly in the form of alkalinity in water bodies. When hydroxides dissolve in water, they increase the pH of the water, making it more alkaline. This can have significant effects on aquatic life, particularly on sensitive species that are adapted to a specific pH range.
Sodium hydroxide
Sodium hydroxide, otherwise called lye and burning pop, is an inorganic compound with the recipe NaOH. It is a white strong ionic compound comprising of sodium cations Na+ and hydroxide anions OH−.
Sodium hydroxide is a profoundly destructive base and salt that decays proteins at common surrounding temperatures and may cause serious substance consumes. It is profoundly dissolvable in water, and promptly retains dampness and carbon dioxide from the air. It frames a progression of hydrates NaOH·nH2O. The monohydrate NaOH·H2O solidifies from water arrangements somewhere in the range of 12.3 and 61.8 °C. The economically accessible “sodium hydroxide” is many times this monohydrate, and distributed information might allude to it rather than the anhydrous compound.
As perhaps of the most straightforward hydroxide, sodium hydroxide is every now and again utilized close by unbiased water and acidic hydrochloric corrosive to exhibit the pH scale to science understudies.
Sodium hydroxide is utilized in numerous businesses: in the creation of wood mash and paper, materials, drinking water, cleansers and cleansers, and as a channel more clean. Overall creation in 2004 was roughly 60 million tons, while request was 51 million tons.
Magnesium hydroxide
Magnesium hydroxide is the inorganic compound with the synthetic recipe Mg(OH)2. It happens in nature as the mineral brucite. It is a white strong with low solvency in water (Ksp = 5.61×10−12). Magnesium hydroxide is a typical part of acid neutralizers, like milk of magnesia.
Potassium hydroxide

Potassium hydroxide is an inorganic compound with the recipe KOH, and is normally called burning potash.
Alongside sodium hydroxide (NaOH), KOH is a prototypical solid base. It has numerous modern and specialty applications, the majority of which exploit its harsh nature and its reactivity toward acids. An expected 700,000 to 800,000 tons were delivered in 2005. KOH is essential as the forerunner to most delicate and fluid cleansers, as well as various potassium-containing synthetics. A white strong is hazardously destructive.
Calcium hydroxide

Calcium hydroxide (customarily called slaked lime) is an inorganic compound with the synthetic equation Ca(OH)2. It is a drab gem or white powder and is created when quicklime (calcium oxide) is blended in with water. It has many names including hydrated lime, burning lime, manufacturers’ lime, slaked lime, cal, and pickling lime. Calcium hydroxide is utilized in numerous applications, including food arrangement, where it has been recognized as E number E526. Limewater, likewise called milk of lime, is the normal name for a soaked arrangement of calcium hydroxide.
Zinc hydroxide
Zinc hydroxide Zn(OH)2 is an inorganic synthetic compound. It additionally happens normally as 3 uncommon minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal).
Like the hydroxides of different metals, like lead, aluminum, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide), is amphoteric. In this manner it will break up promptly in a weaken arrangement of a solid corrosive, like HCl, and furthermore in an answer of a salt like sodium hydroxide.
Iron(II) hydroxide
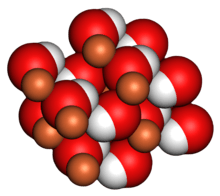
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the recipe Fe(OH)2. It is created when iron(II) salts, from a compound like iron(II) sulfate, are treated with hydroxide particles. Iron(II) hydroxide is a white strong, yet even hints of oxygen give a greenish hint. The air-oxidized strong is at times known as “green rust”.
White paper on Hydroxides
Certainly! Here is a white paper on hydroxides:
Introduction
Hydroxides are inorganic compounds that contain a metal cation and a hydroxide anion (OH-). They are a type of base that reacts with acids to form water and a salt. Hydroxides have a wide range of applications in various industries, including chemicals, agriculture, medicine, and wastewater treatment.
Properties and Structure
The properties of hydroxides can vary widely depending on the specific compound. In general, they are usually solid, crystalline substances with a high melting point. They are also typically soluble in water and have a high pH (alkaline) when dissolved.
The structure of hydroxides is typically ionic, with the metal cation and hydroxide anion held together by electrostatic forces. The hydroxide anion consists of one oxygen atom and one hydrogen atom, bonded covalently. The oxygen atom has a partial negative charge, while the hydrogen atom has a partial positive charge. This gives the hydroxide anion an overall negative charge, which is balanced by the positive charge of the metal cation.
Applications
Hydroxides have a wide range of applications in various industries. Some examples include:
- Chemicals: Hydroxides are used in the production of many chemicals, including soaps, detergents, and fertilizers. Sodium hydroxide (NaOH), also known as caustic soda, is one of the most widely used hydroxides in the chemical industry.
- Agriculture: Hydroxides are used in agriculture to adjust the pH of soils and to treat acidic soils. Calcium hydroxide (Ca(OH)2), also known as slaked lime, is commonly used for this purpose.
- Medicine: Hydroxides are used in medicine as antacids to neutralize stomach acid. Magnesium hydroxide (Mg(OH)2), also known as milk of magnesia, is a commonly used antacid.
- Wastewater Treatment: Hydroxides are used in wastewater treatment to neutralize acidic wastewater and to remove heavy metals from the water. Calcium hydroxide is often used for this purpose.
Environmental Implications
Hydroxides can have important environmental implications, particularly in the form of alkalinity in water bodies. When hydroxides dissolve in water, they increase the pH of the water, making it more alkaline. This can have significant effects on aquatic life, particularly on sensitive species that are adapted to a specific pH range. For this reason, it is important to carefully manage the discharge of hydroxides into water bodies.
Conclusion
Hydroxides are inorganic compounds that have a wide range of applications in various industries. They are typically solid, crystalline substances with a high melting point and are soluble in water. Hydroxides have important environmental implications, particularly in the form of alkalinity in water bodies. For this reason, it is important to carefully manage the use and disposal of hydroxides in order to minimize their impact on the environment.
