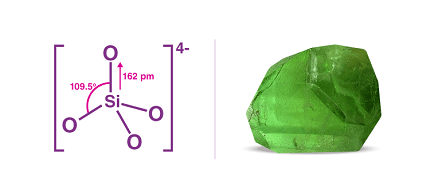
Group 14 silicates are a subgroup of silicate minerals that contain tetrahedral SiO4 units linked together to form a three-dimensional framework. The group includes several important minerals, including quartz, feldspar, and mica.
The SiO4 tetrahedra in group 14 silicates are linked together in a way that creates a continuous network. The bonding between the tetrahedra is strong, and this gives the minerals their hardness and durability. The structure of group 14 silicates is characterized by sheets of tetrahedra linked together by oxygen ions.
The most common minerals in this group are quartz, which is composed entirely of SiO4 tetrahedra, and feldspar, which contains a combination of SiO4 tetrahedra and AlO4 tetrahedra. Mica is also a member of this group, and it contains sheets of tetrahedra linked by potassium or other cations.
Group 14 silicates have a wide range of physical and chemical properties, depending on their composition and structure. Quartz, for example, is hard, durable, and resistant to chemical weathering, making it a valuable mineral in a variety of industrial applications. Feldspar is also widely used in industry, particularly in the manufacture of ceramics and glass.
Overall, group 14 silicates are an important group of minerals that play a significant role in many aspects of human life, from construction and industry to electronics and telecommunications.
What is Required p-Block Elements Group 14 Silicates
The required p-block elements for the formation of group 14 silicates are silicon (Si) and germanium (Ge). These elements are located in group 14 of the periodic table and have four valence electrons, which allows them to form the tetrahedral SiO4 units that are characteristic of group 14 silicates.
Silicon is the most abundant element in the Earth’s crust and is the primary component of many minerals, including quartz and feldspar. It is also an essential component of many technological applications, including semiconductors, solar cells, and computer chips.
Germanium is a less abundant element that is similar in many respects to silicon. It has a higher density and melting point than silicon, and it is also used in some electronic applications, such as infrared detectors and high-performance transistors.
Other elements in group 14, including tin (Sn) and lead (Pb), can also form silicates under certain conditions, but they are less common than silicon and germanium. Overall, the group 14 elements play an important role in the formation of silicates and other minerals, as well as in many technological applications.
Who is Required p-Block Elements Group 14 Silicates
The required p-block elements for the formation of group 14 silicates are silicon (Si) and germanium (Ge). These elements are located in group 14 of the periodic table and are characterized by having four valence electrons in their outermost shell.
Silicon is the second most abundant element in the Earth’s crust, making up approximately 28% of its mass. It is widely used in industry, particularly in the manufacture of semiconductors, glass, and ceramics. Silicon is also an essential component of many electronic devices, including solar cells, computer chips, and LEDs.
Germanium is a less abundant element than silicon, but it is still an important material for electronic applications. It has a higher density and melting point than silicon, and it is used in some high-performance transistors and infrared detectors.
Other elements in group 14, including tin (Sn) and lead (Pb), can also form silicates under certain conditions, but they are less common than silicon and germanium.
Overall, the group 14 elements play an important role in the formation of silicates and other minerals, as well as in many technological applications.
When is Required p-Block Elements Group 14 Silicates
The required p-block elements for the formation of group 14 silicates have been present in the Earth’s crust since its formation. Silicon (Si) and germanium (Ge), the two primary elements involved in the formation of group 14 silicates, were formed through the nuclear fusion processes that occurred in the early universe and were subsequently distributed throughout the galaxy as part of the materials that formed our solar system.
The Earth’s crust has been evolving over billions of years, with the formation and transformation of minerals influenced by geological processes such as tectonic activity, erosion, and weathering. The formation of group 14 silicates such as quartz and feldspar involves the interaction of silicon and oxygen in the Earth’s crust, and this process has been ongoing since the formation of the planet.
Silicon and germanium have also been widely used by humans in various technological applications, particularly in the past century with the rise of electronics and information technology. The demand for these elements has increased with the development of new technologies and will likely continue to do so in the future.
Where is Required p-Block Elements Group 14 Silicates
The required p-block elements for the formation of group 14 silicates, silicon (Si) and germanium (Ge), are abundant in the Earth’s crust. Silicon is the second most abundant element in the Earth’s crust, making up about 28% of its mass, while germanium is much less abundant.
Group 14 silicates are found in a wide range of geological environments, including igneous, sedimentary, and metamorphic rocks. Quartz, one of the most common group 14 silicates, can be found in many different types of rocks, including granite, sandstone, and shale. Feldspar is also a common group 14 silicate and can be found in igneous and metamorphic rocks, as well as in sediments.
In addition to their natural occurrence, silicon and germanium are also widely used by humans in various technological applications. Silicon, in particular, is a critical component in the manufacturing of semiconductors, which are used in electronic devices such as computer chips and solar cells. Germanium is also used in electronics, as well as in infrared detectors and high-performance transistors.
Overall, the required p-block elements for the formation of group 14 silicates are widely distributed throughout the Earth’s crust and have played an important role in both natural geological processes and human technological advancements.
How is Required p-Block Elements Group 14 Silicates
The required p-block elements for the formation of group 14 silicates, silicon (Si) and germanium (Ge), are involved in the formation of minerals through a complex series of geological processes. Group 14 silicates are formed by the combination of silicon and oxygen in tetrahedral structures, with other elements such as aluminum, potassium, or sodium often replacing some of the silicon atoms.
The formation of group 14 silicates occurs through a variety of geological processes, including volcanic activity, weathering, and sedimentation. For example, magma from volcanic activity can crystallize to form igneous rocks containing quartz and feldspar. Over time, these rocks can be weathered and eroded, releasing the silicates into sediments that eventually become sedimentary rocks. Under conditions of high pressure and temperature, these rocks can be metamorphosed, transforming the silicates into new minerals.
The formation of group 14 silicates has also been influenced by human activity, particularly in the past century with the rise of electronics and information technology. Silicon, in particular, is a critical component in the manufacturing of semiconductors, which are used in electronic devices such as computer chips and solar cells. The production of high-quality silicon for these applications involves a variety of industrial processes, including purification and crystal growth.
Overall, the formation and use of group 14 silicates involve a complex interplay between geological and technological processes, and their properties and applications have been shaped by millions of years of natural evolution as well as the ingenuity of humans.
Case Study on p-Block Elements Group 14 Silicates
One interesting case study involving p-block elements group 14 silicates is the use of silicon in the production of solar panels. Solar panels, which are used to convert sunlight into electricity, typically consist of silicon-based photovoltaic cells.
The photovoltaic effect, which is used to generate electricity in solar panels, involves the conversion of light energy into electrical energy. This process is facilitated by the properties of silicon, which has a unique electronic structure that allows it to absorb and release photons of light energy.
To produce solar panels, high-quality silicon must be first produced through a series of industrial processes. The initial step is the purification of silicon from raw materials such as silica sand or quartzite. This involves the reduction of silicon dioxide to pure silicon using a reducing agent such as carbon. The resulting silicon is then further purified using various techniques, including zone melting and chemical vapor deposition.
Once the purified silicon has been obtained, it is then processed into wafers using techniques such as slicing, polishing, and etching. These wafers are then doped with other elements such as boron or phosphorus to create the p-type and n-type semiconductors that are used in the photovoltaic cells.
The photovoltaic cells are then assembled into solar panels, which can be used to generate electricity from sunlight. The use of silicon in solar panel production has revolutionized the renewable energy industry, allowing for the widespread adoption of solar power as a clean and sustainable source of energy.
Overall, this case study highlights the importance of p-block elements group 14 silicates, particularly silicon, in technological applications that have the potential to revolutionize our energy systems and reduce our reliance on fossil fuels.
White paper on p-Block Elements Group 14 Silicates
Introduction
Group 14 silicates, consisting of silicon (Si) and germanium (Ge), are essential elements in various geological and technological applications. These elements are abundant in the Earth’s crust and have unique properties that make them critical components in a wide range of materials, including semiconductors, glass, and ceramics. This white paper aims to provide an overview of the properties, applications, and future prospects of p-block elements group 14 silicates.
Properties of Group 14 Silicates
Group 14 silicates are characterized by their tetrahedral structures, consisting of silicon or germanium atoms surrounded by four oxygen atoms. These structures give rise to a range of properties, including high melting and boiling points, high electrical resistivity, and excellent thermal stability. These properties make group 14 silicates ideal for use in materials that require high strength and durability.
Applications of Group 14 Silicates
Group 14 silicates have a wide range of applications in various industries, including electronics, construction, and automotive. Silicon, in particular, is a critical component in the manufacturing of semiconductors, which are used in electronic devices such as computer chips and solar cells. The use of silicon in solar panels has revolutionized the renewable energy industry, allowing for the widespread adoption of solar power as a clean and sustainable source of energy.
Group 14 silicates are also commonly used in the production of glass and ceramics. For example, silica, which is a type of group 14 silicate, is used to produce glass for windows and lenses, as well as in the manufacture of ceramics such as porcelain and stoneware. Group 14 silicates are also used as fillers and reinforcing agents in polymers and composites, providing enhanced mechanical and thermal properties.
Future Prospects
The future prospects for group 14 silicates are bright, with ongoing research focusing on the development of new materials and technologies. In particular, the use of group 14 silicates in energy storage and conversion technologies such as batteries and fuel cells is an area of active research. Additionally, the development of novel applications for group 14 silicates, such as in biomedical implants and nanotechnology, is a promising avenue for future exploration.
Conclusion
Group 14 silicates, consisting of silicon and germanium, are essential elements in various geological and technological applications. These elements have unique properties that make them critical components in materials used in electronics, construction, and automotive industries. Ongoing research in the field of group 14 silicates is likely to lead to the development of new materials and technologies that will further expand their applications and potential impact on society.
