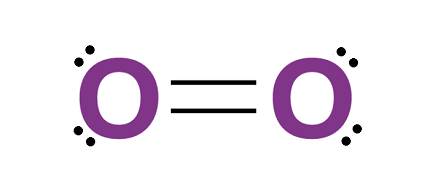
Dioxygen, also known as molecular oxygen or simply oxygen, is a gas that makes up about 21% of Earth’s atmosphere. It is an essential element for life as we know it, and has many important uses in various fields. Here are some of the uses of dioxygen:
- Respiration: Oxygen is essential for the process of respiration in living organisms, including humans. It is used by the body to produce energy from food through a process called cellular respiration.
- Combustion: Oxygen supports combustion, which is the process of burning. It is required for most types of fire, such as in fuel-powered engines, heating systems, and cooking appliances.
- Oxidation: Oxygen is a highly reactive element and is used in many oxidation processes, including rusting, bleaching, and sterilization. It is also used in the production of many chemicals, such as hydrogen peroxide.
- Welding: Oxygen is used in welding and cutting processes, where it is combined with a fuel gas to produce a high-temperature flame that melts the metal to be welded or cut.
- Medical uses: Oxygen is used in medical applications to treat breathing disorders and provide respiratory support. It is also used in anesthesia and in hyperbaric oxygen therapy.
- Water treatment: Oxygen is used in water treatment plants to remove impurities and kill bacteria and other harmful organisms.
- Ozone production: Oxygen is used to produce ozone, a highly reactive gas that is used in many industrial and medical applications, including water treatment, disinfection, and air purification.
- Space exploration: Oxygen is a critical component of life support systems used in space exploration, providing breathable air for astronauts and supporting combustion in rocket engines.
Overall, dioxygen is an incredibly versatile element that has many important uses in various fields, ranging from life support to industrial processes.
What is Required p-Block Elements Group 16 Uses of dioxygen
The p-block elements in Group 16, also known as the chalcogens, have unique properties that make them particularly useful in conjunction with dioxygen. Here are some of the uses of dioxygen with Group 16 elements:
- Sulfur: Sulfur is a Group 16 element that is commonly used in the production of sulfuric acid, which is a key industrial chemical used in the production of fertilizers, dyes, and detergents. Sulfur is also used in the vulcanization of rubber and in the production of matches.
- Oxygen scavengers: Some Group 16 elements, such as sulfur and selenium, are used as oxygen scavengers in industrial processes to prevent the oxidation of metals and other materials. This helps to prevent corrosion and other forms of degradation.
- Oxygen carriers: Certain Group 16 elements, such as tellurium and polonium, are used as oxygen carriers in industrial processes, such as the production of semiconductors and nuclear batteries.
- Oxygen sensors: Group 16 elements, particularly sulfur and selenium, are used in oxygen sensors to detect the presence of oxygen in various environments, including medical and industrial settings.
- Pharmaceuticals: Certain Group 16 elements, such as sulfur and selenium, have medicinal properties and are used in the production of pharmaceuticals, such as antibiotics and anti-inflammatory drugs.
- Cosmetics: Group 16 elements, such as sulfur and selenium, are used in cosmetics and personal care products due to their antibacterial and antifungal properties.
- Energy storage: Certain Group 16 elements, such as sulfur, are being investigated for their potential use in advanced energy storage technologies, such as lithium-sulfur batteries.
In summary, Group 16 elements have a wide range of uses in conjunction with dioxygen, including in industrial processes, medical and pharmaceutical applications, and energy storage.
When is Required p-Block Elements Group 16 Uses of dioxygen
The uses of p-block elements in Group 16 with dioxygen have been observed and studied for many years, and their applications continue to evolve as new technologies and industries emerge. These uses are ongoing and have been established through extensive research and experimentation in various fields, including chemistry, medicine, industry, and energy storage. Some of these uses, such as the production of sulfuric acid and the use of sulfur in vulcanizing rubber, have been around for over a century. Others, such as the use of sulfur in lithium-sulfur batteries, are more recent and are still in the development and testing phases. In summary, the uses of p-block elements in Group 16 with dioxygen are ongoing and continue to evolve as new applications and technologies emerge.
Where is Required p-Block Elements Group 16 Uses of dioxygen
The uses of p-block elements in Group 16 with dioxygen can be found in various industries and applications around the world. Here are some examples:
- Industrial processes: Sulfuric acid, which is produced using sulfur, is a key industrial chemical used in the production of fertilizers, dyes, and detergents. It is used in many industries, including agriculture, textiles, and mining.
- Energy storage: Sulfur is being investigated as a potential material for use in advanced energy storage technologies, such as lithium-sulfur batteries. This research is being conducted in universities and research institutions around the world.
- Medical and pharmaceutical applications: Sulfur and other Group 16 elements are used in the production of pharmaceuticals, such as antibiotics and anti-inflammatory drugs, and in medical devices, such as oxygen sensors.
- Cosmetics and personal care products: Sulfur and other Group 16 elements are used in cosmetics and personal care products for their antibacterial and antifungal properties.
- Semiconductors and electronics: Tellurium, a Group 16 element, is used in the production of semiconductors and other electronic devices.
Overall, the uses of p-block elements in Group 16 with dioxygen can be found in a wide range of industries and applications around the world, from agriculture and mining to electronics and energy storage.
How is Required p-Block Elements Group 16 Uses of dioxygen
The uses of p-block elements in Group 16 with dioxygen are varied and depend on the specific element and application. Here are some examples of how these elements are used with dioxygen:
- Production of sulfuric acid: Sulfur is burned in the presence of dioxygen to produce sulfur dioxide gas, which is then converted to sulfur trioxide in the presence of a catalyst and more dioxygen. The resulting sulfur trioxide is then used to produce sulfuric acid, which is a key industrial chemical.
- Oxygen scavengers: In industrial processes, Group 16 elements such as sulfur and selenium are used as oxygen scavengers to prevent the oxidation of metals and other materials. The elements react with dioxygen to form oxides or other compounds, preventing the oxygen from reacting with the materials.
- Oxygen carriers: Tellurium and polonium, Group 16 elements, are used as oxygen carriers in certain industrial processes. They react with dioxygen to form oxides, which are then used in the production of semiconductors and other electronic devices.
- Oxygen sensors: Sulfur and selenium, Group 16 elements, are used in oxygen sensors to detect the presence of dioxygen in various environments. These elements react with dioxygen to form oxides, which can then be measured to determine the oxygen concentration.
- Energy storage: Sulfur is being investigated as a potential material for use in advanced energy storage technologies, such as lithium-sulfur batteries. In these batteries, sulfur reacts with dioxygen to form lithium sulfide and other compounds, releasing energy that can be used to power devices.
Overall, the uses of p-block elements in Group 16 with dioxygen involve various chemical reactions and processes that are carefully controlled to achieve the desired outcome. These elements and their applications have been studied extensively and continue to evolve as new technologies and industries emerge.
Nomenclature of p-Block Elements Group 16 Uses of dioxygen
The p-block elements in Group 16 have specific names based on their atomic number and electronic configuration. Here are the names and symbols of the elements in Group 16:
- Oxygen – O
- Sulfur – S
- Selenium – Se
- Tellurium – Te
- Polonium – Po
These names are based on the International Union of Pure and Applied Chemistry (IUPAC) naming conventions for chemical elements. The symbols are abbreviations that are used to represent the elements in chemical formulas and equations.
In addition to these basic names, there are also common names for some of these elements. For example, sulfur is sometimes referred to as brimstone, and tellurium is sometimes called “metallic wood” because of its silvery-white color and brittle texture.
Overall, the nomenclature of p-block elements in Group 16 is based on their unique atomic structure and properties, and these names are used in various scientific and industrial applications to describe and identify these elements.
Case Study on p-Block Elements Group 16 Uses of dioxygen
One case study on the uses of p-block elements in Group 16 with dioxygen is the development of lithium-sulfur batteries, which are a potential alternative to lithium-ion batteries for energy storage.
Sulfur is a Group 16 element that can react with dioxygen to form sulfur dioxide, sulfur trioxide, and other compounds. In a lithium-sulfur battery, sulfur is used as the cathode material, while lithium is used as the anode. When the battery is discharged, sulfur reacts with lithium ions and dioxygen to form lithium sulfide and other compounds, releasing energy that can be used to power devices.
Lithium-sulfur batteries have several advantages over lithium-ion batteries, including a higher energy density, lower cost, and lower environmental impact. However, they also face several challenges, such as poor cycle life, low efficiency, and the formation of insoluble reaction products that can degrade the performance of the battery.
Researchers around the world are working to address these challenges and improve the performance of lithium-sulfur batteries. One approach is to use nanostructured sulfur materials that can improve the stability and efficiency of the battery. Another approach is to use additives, such as Group 16 elements like selenium and tellurium, to improve the electrochemical performance of the battery.
Overall, the development of lithium-sulfur batteries is an example of how p-block elements in Group 16 can be used with dioxygen to create new and innovative technologies for energy storage. This case study highlights the importance of continued research and development in this field to improve the performance and viability of these batteries for practical applications.
White paper on p-Block Elements Group 16 Uses of dioxygen
White Paper: p-Block Elements Group 16 Uses of Dioxygen
Introduction:
The p-block elements in Group 16 of the periodic table are versatile and have a wide range of uses when combined with dioxygen. These elements include oxygen, sulfur, selenium, tellurium, and polonium. In this white paper, we will explore the various uses of these elements with dioxygen, including their applications in industrial processes, energy storage, and electronic devices.
Production of Sulfuric Acid:
One of the most important industrial applications of p-block elements in Group 16 with dioxygen is the production of sulfuric acid. Sulfur is burned in the presence of dioxygen to produce sulfur dioxide gas, which is then converted to sulfur trioxide in the presence of a catalyst and more dioxygen. The resulting sulfur trioxide is then used to produce sulfuric acid, which is a key industrial chemical used in the production of fertilizers, detergents, and other products.
Oxygen Scavengers:
Group 16 elements such as sulfur and selenium are used as oxygen scavengers in industrial processes to prevent the oxidation of metals and other materials. The elements react with dioxygen to form oxides or other compounds, preventing the oxygen from reacting with the materials. This is important for preventing corrosion, extending the lifespan of materials, and improving the efficiency of industrial processes.
Oxygen Carriers:
Tellurium and polonium, Group 16 elements, are used as oxygen carriers in certain industrial processes. They react with dioxygen to form oxides, which are then used in the production of semiconductors and other electronic devices. For example, tellurium oxide is used in the production of rewritable optical discs and as a component in solar cells.
Oxygen Sensors:
Sulfur and selenium, Group 16 elements, are used in oxygen sensors to detect the presence of dioxygen in various environments. These elements react with dioxygen to form oxides, which can then be measured to determine the oxygen concentration. Oxygen sensors are used in a variety of applications, including medical devices, automotive systems, and industrial processes.
Energy Storage:
Sulfur is being investigated as a potential material for use in advanced energy storage technologies, such as lithium-sulfur batteries. In these batteries, sulfur reacts with dioxygen to form lithium sulfide and other compounds, releasing energy that can be used to power devices. Lithium-sulfur batteries have several advantages over lithium-ion batteries, including a higher energy density and lower cost.
Conclusion:
The p-block elements in Group 16 have many important uses when combined with dioxygen, including the production of sulfuric acid, oxygen scavenging, oxygen carriers, oxygen sensors, and energy storage. These elements are critical to a wide range of industrial processes, electronic devices, and energy technologies, and their continued research and development is essential for continued progress in these fields. As scientists continue to explore the properties and applications of these elements, we can expect to see new and innovative uses of p-block elements in Group 16 with dioxygen emerge in the future.
