Sulfur forms a variety of oxoacids, which are acids that contain oxygen and sulfur. There are several oxoacids of sulfur, but the most common ones are the Group 16 oxoacids of sulfur, which include sulfuric acid, sulfurous acid, thiosulfuric acid, and dithionic acid.
- Sulfuric acid (H2SO4): This is the most commonly used oxoacid of sulfur. It is a strong acid and is commonly used in the production of fertilizers, detergents, and other chemicals. Sulfuric acid is also used as an electrolyte in lead-acid batteries.
- Sulfurous acid (H2SO3): This is a weaker acid than sulfuric acid and is commonly used as a reducing agent. It is also used in the production of paper, textiles, and leather.
- Thiosulfuric acid (H2S2O3): This acid is used in the photographic industry as a fixing agent. It is also used as a reducing agent and in the production of chemicals.
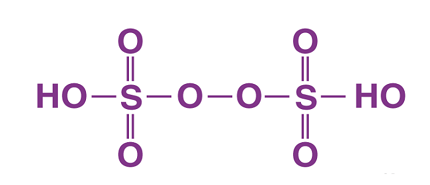
- Dithionic acid (H2S2O6): This acid is used as a bleaching agent and in the production of detergents. It is also used in the textile industry to remove excess dye from fabrics.
All of these oxoacids of sulfur have important industrial and commercial applications, and they are also important in the study of chemistry and chemical reactions.
What is Required p-Block Elements Group 16 Oxoacids of sulfur
The p-block elements that are required for the formation of the Group 16 oxoacids of sulfur are sulfur (S), selenium (Se), and tellurium (Te). These elements are in Group 16 of the periodic table, also known as the chalcogens.
Sulfur, selenium, and tellurium can form a variety of oxoacids depending on the number of oxygen atoms present. The oxoacids of sulfur, as mentioned earlier, include sulfuric acid (H2SO4), sulfurous acid (H2SO3), thiosulfuric acid (H2S2O3), and dithionic acid (H2S2O6).
Similarly, selenium and tellurium can also form oxoacids such as selenious acid (H2SeO3), selenic acid (H2SeO4), tellurous acid (H2TeO3), and telluric acid (H2TeO4), among others.
The properties of these oxoacids depend on the oxidation state of the element, the number of oxygen atoms present, and the strength of the bond between the element and the oxygen atom. The Group 16 oxoacids of sulfur are particularly important due to their widespread industrial and commercial applications.
When is Required p-Block Elements Group 16 Oxoacids of sulfur
The Group 16 oxoacids of sulfur, as well as other oxoacids of chalcogens (elements in Group 16 of the periodic table), are required in a variety of applications in industry, research, and everyday life.
Sulfuric acid, for example, is widely used in the production of fertilizers, detergents, and other chemicals, as well as in the processing of metals, such as the extraction of copper and zinc. Sulfuric acid is also used as an electrolyte in lead-acid batteries and in the production of synthetic fibers and plastics.
Sulfurous acid is used as a reducing agent in a variety of chemical reactions, as well as in the production of paper, textiles, and leather.
Thiosulfuric acid is used in the photographic industry as a fixing agent, as well as in the production of chemicals.
Dithionic acid is used as a bleaching agent and in the production of detergents, as well as in the textile industry to remove excess dye from fabrics.
Selenium and tellurium oxoacids also have important applications in industry and research, such as in the production of glass and ceramics, the manufacturing of semiconductors, and in the development of catalysts.
In summary, the Group 16 oxoacids of sulfur and other chalcogens are required for a variety of applications in industry and research and play an important role in many everyday products and processes.
Where is Required p-Block Elements Group 16 Oxoacids of sulfur
The Group 16 oxoacids of sulfur and other chalcogens are widely used in many industries and applications worldwide. They can be found in various locations, including:
- Chemical plants: Sulfuric acid, sulfurous acid, thiosulfuric acid, and dithionic acid are commonly used in chemical plants for the production of fertilizers, detergents, and other chemicals.
- Mining operations: Sulfuric acid is used in the processing of metals, such as copper and zinc, in mining operations.
- Batteries: Sulfuric acid is used as an electrolyte in lead-acid batteries, which are commonly used in vehicles and other applications.
- Textile industry: Dithionic acid is used in the textile industry as a bleaching agent and to remove excess dye from fabrics.
- Photography: Thiosulfuric acid is used in the photographic industry as a fixing agent.
- Water treatment: Sulfuric acid is used in water treatment to adjust the pH level and to remove impurities.
- Semiconductor industry: Selenium and tellurium oxoacids are used in the semiconductor industry to manufacture electronic components.
These are just a few examples of where the Group 16 oxoacids of sulfur and other chalcogens can be found. They are essential in many industrial processes and are used in a wide variety of applications.
How is Required p-Block Elements Group 16 Oxoacids of sulfur
The Group 16 oxoacids of sulfur and other chalcogens are typically produced through chemical reactions involving the respective element and oxygen or other oxidizing agents. The process and conditions for their production depend on the specific oxoacid being produced. Here are a few examples:
- Sulfuric acid (H2SO4): Sulfuric acid is typically produced through the contact process, which involves the reaction of sulfur dioxide (SO2) and oxygen (O2) in the presence of a catalyst to form sulfur trioxide (SO3). The SO3 is then reacted with water (H2O) to form sulfuric acid. Alternatively, sulfuric acid can be produced through the wet sulfuric acid process, which involves the reaction of sulfur dioxide with water and an oxidizing agent such as nitric acid (HNO3).
- Sulfurous acid (H2SO3): Sulfurous acid can be produced by dissolving sulfur dioxide in water. This reaction is typically carried out in the presence of a catalyst, such as hydrogen peroxide (H2O2).
- Thiosulfuric acid (H2S2O3): Thiosulfuric acid is typically produced by the reaction of sulfur dioxide with sodium thiosulfate (Na2S2O3) in the presence of hydrochloric acid (HCl).
- Dithionic acid (H2S2O6): Dithionic acid is typically produced through the reaction of sulfuric acid with hydrogen peroxide.
The production of selenium and tellurium oxoacids follows similar principles, involving the respective element and oxygen or other oxidizing agents.
Overall, the production of Group 16 oxoacids of sulfur and other chalcogens involves careful control of reaction conditions, including temperature, pressure, and reactant concentrations, to ensure high yields and purity of the final product.
Production of p-Block Elements Group 16 Oxoacids of sulfur
The Group 16 oxoacids of sulfur, including sulfuric acid (H2SO4), sulfurous acid (H2SO3), thiosulfuric acid (H2S2O3), and dithionic acid (H2S2O6), are typically produced through chemical processes involving the respective element and oxygen or other oxidizing agents.
Here are some general methods for producing the Group 16 oxoacids of sulfur:
- Sulfuric acid: The contact process is the most common method for producing sulfuric acid. This process involves the reaction of sulfur dioxide (SO2) and oxygen (O2) in the presence of a catalyst, typically vanadium pentoxide (V2O5), to produce sulfur trioxide (SO3). The SO3 is then absorbed in water to form sulfuric acid. The reaction is exothermic and requires careful control of temperature and pressure to prevent the formation of undesirable by-products, such as sulfur dioxide.
- Sulfurous acid: Sulfurous acid is typically produced by dissolving sulfur dioxide (SO2) in water. This reaction is exothermic and can be accelerated by the addition of a catalyst, such as hydrogen peroxide (H2O2).
- Thiosulfuric acid: Thiosulfuric acid can be produced by reacting sulfur dioxide (SO2) with sodium thiosulfate (Na2S2O3) in the presence of hydrochloric acid (HCl). The reaction produces sulfur dioxide gas and thiosulfuric acid, which can be purified by distillation.
- Dithionic acid: Dithionic acid is typically produced by reacting sulfuric acid (H2SO4) with hydrogen peroxide (H2O2). The reaction is exothermic and requires careful temperature control to prevent the formation of undesirable by-products.
These methods can be adapted for the production of other oxoacids of chalcogens in Group 16, such as selenium and tellurium oxoacids. The specific reaction conditions and catalysts used will depend on the specific oxoacid being produced and the desired yield and purity of the final product.
Case Study on p-Block Elements Group 16 Oxoacids of sulfur
One important application of p-Block Elements Group 16 Oxoacids of sulfur is in the production of fertilizers, particularly ammonium sulfate (NH4)2SO4, which is used extensively in agriculture.
Ammonium sulfate is typically produced through a reaction between sulfuric acid (H2SO4) and ammonia gas (NH3). The sulfuric acid is first produced through the contact process, which involves the reaction of sulfur dioxide (SO2) and oxygen (O2) in the presence of a catalyst to form sulfur trioxide (SO3). The SO3 is then reacted with water (H2O) to form sulfuric acid. The ammonia gas is produced through the Haber-Bosch process, which involves the reaction of nitrogen gas (N2) and hydrogen gas (H2) in the presence of a catalyst, typically iron or nickel, at high temperature and pressure.
The ammonium sulfate production process can be summarized as follows:
- Production of sulfuric acid: Sulfur dioxide (SO2) is reacted with oxygen (O2) in the presence of a catalyst, typically vanadium pentoxide (V2O5), to produce sulfur trioxide (SO3). The SO3 is then absorbed in water (H2O) to form sulfuric acid (H2SO4).
- Production of ammonia: Nitrogen gas (N2) and hydrogen gas (H2) are reacted in the presence of a catalyst, typically iron or nickel, at high temperature and pressure to produce ammonia gas (NH3).
- Reaction of sulfuric acid and ammonia: The sulfuric acid and ammonia gas are reacted to produce ammonium sulfate ((NH4)2SO4).
(NH3 + H2SO4 -> (NH4)2SO4)
The resulting ammonium sulfate can be dried and granulated for use as a fertilizer. Ammonium sulfate provides a readily available source of nitrogen and sulfur for plant growth, and is often used in conjunction with other fertilizers to promote optimal crop yields.
In addition to its use in fertilizer production, sulfuric acid is also widely used in a variety of industrial applications, such as the production of detergents, dyes, and pharmaceuticals. Sulfurous acid, thiosulfuric acid, and dithionic acid also find use in various chemical applications, such as in the production of photographic chemicals and as reducing agents in chemical synthesis.
Overall, the Group 16 oxoacids of sulfur play a critical role in a wide range of industrial and agricultural applications, and their production and use continue to be an important area of research and development in chemistry and chemical engineering.
White paper on p-Block Elements Group 16 Oxoacids of sulfur
Introduction:
The p-Block Elements Group 16 Oxoacids of sulfur are an important group of chemical compounds with numerous industrial and agricultural applications. This white paper will provide an overview of the properties, production methods, and applications of these compounds.
Properties:
The p-Block Elements Group 16 Oxoacids of sulfur include sulfuric acid (H2SO4), sulfurous acid (H2SO3), thiosulfuric acid (H2S2O3), and dithionic acid (H2S2O6). These compounds are all acidic in nature, with varying degrees of acidity depending on the number of oxygen atoms present in the molecule. Sulfuric acid is the most commonly used and is a strong acid, while sulfurous acid is a weak acid.
Production:
The production of these oxoacids typically involves the reaction of the respective element and oxygen or other oxidizing agents. For example, sulfuric acid is typically produced through the contact process, which involves the reaction of sulfur dioxide (SO2) and oxygen (O2) in the presence of a catalyst to form sulfur trioxide (SO3). The SO3 is then reacted with water (H2O) to form sulfuric acid. Other oxoacids such as thiosulfuric acid and dithionic acid are typically produced through chemical reactions between sulfuric acid and other compounds, such as sodium thiosulfate or hydrogen peroxide.
Applications:
The p-Block Elements Group 16 Oxoacids of sulfur have numerous industrial and agricultural applications. Sulfuric acid is one of the most widely used industrial chemicals and is used in the production of a variety of products, including detergents, fertilizers, and pharmaceuticals. It is also used as a catalyst in numerous chemical reactions. Sulfurous acid is used in the production of wine and other food products, as well as in the manufacture of paper and textiles.
Thiosulfuric acid and dithionic acid are used in various chemical applications, such as in the production of photographic chemicals and as reducing agents in chemical synthesis. Thiosulfuric acid is also used as a bleaching agent in the paper and textile industries.
Conclusion:
In conclusion, the p-Block Elements Group 16 Oxoacids of sulfur are an important group of chemical compounds with numerous industrial and agricultural applications. Their properties, production methods, and applications make them a critical component of many chemical processes, and ongoing research continues to explore new ways to optimize their production and use. As such, understanding the properties and applications of these oxoacids is crucial for the advancement of modern chemistry and chemical engineering.
