Group 17 of the periodic table, also known as the halogens, consists of five non-metallic elements: fluorine, chlorine, bromine, iodine, and astatine. Here are some general methods for the preparation and manufacture of these elements:
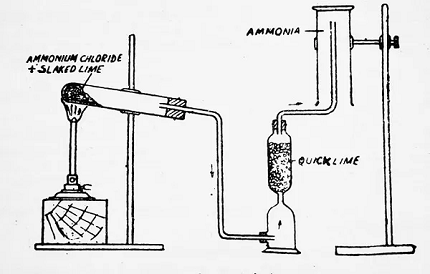
Fluorine: Fluorine is the most reactive of all the halogens and is typically produced by the electrolysis of a mixture of hydrofluoric acid and potassium fluoride. Alternatively, it can also be produced by the reaction of hydrogen fluoride with elemental fluorine.
Chlorine: Chlorine is usually prepared by the electrolysis of a solution of sodium chloride (brine). This process is called the chloralkali process and involves the production of chlorine gas, hydrogen gas, and sodium hydroxide.
Bromine: Bromine can be obtained by the treatment of a solution of a bromide salt with chlorine gas. The chlorine oxidizes the bromide ion to bromine, which can then be collected by distillation.
Iodine: Iodine can be obtained by the reaction of iodide salts with oxidizing agents such as chlorine or hydrogen peroxide. The iodine is liberated as a solid, which can be collected by filtration and sublimation.
Astatine: Astatine is the rarest naturally occurring element on Earth, and its preparation is a challenging task due to its scarcity and high radioactivity. Astatine can be produced by bombarding bismuth-209 with alpha particles in a cyclotron or nuclear reactor. However, due to its high radioactivity, astatine is primarily used for research purposes only.
Overall, the preparation and manufacture of the halogens require specialized equipment and safety precautions due to the highly reactive and potentially hazardous nature of these elements.
What is Required p-Block Elements Group 17 Preparation/Manufacture
The p-block elements of Group 17, also known as the halogens, require different methods for their preparation and manufacture. Here are some of the common methods used for the preparation of halogens:
Fluorine:
Fluorine is usually produced by the electrolysis of a solution of hydrogen fluoride and potassium fluoride in a metal container. The electrolysis cell contains an anode made of a fluorine-resistant material, such as nickel, and a cathode made of a material that is resistant to hydrofluoric acid, such as platinum. The electrolysis process is carried out at a low temperature and high pressure to prevent the explosive reaction of fluorine gas with other substances.
Chlorine:
Chlorine is generally prepared by the electrolysis of a solution of sodium chloride (brine) in a diaphragm cell. The anode and cathode are separated by a porous diaphragm, which prevents the mixing of chlorine gas with sodium hydroxide solution. Chlorine gas is collected at the anode, while hydrogen gas is collected at the cathode. The chlorine gas is then purified by washing with water and drying with sulfuric acid.
Bromine:
Bromine can be prepared by treating a solution of a bromide salt, such as potassium bromide, with chlorine gas. The chlorine oxidizes the bromide ion to bromine, which is then collected by distillation.
Iodine:
Iodine is typically prepared by the reaction of iodide salts, such as potassium iodide, with oxidizing agents such as hydrogen peroxide or chlorine. The iodine is liberated as a solid, which is then collected by filtration and sublimation.
Astatine:
Astatine is a highly radioactive element, and its preparation is very challenging due to its scarcity and short half-life. Astatine can be produced by bombarding bismuth-209 with alpha particles in a cyclotron or nuclear reactor. However, due to its high radioactivity, astatine is primarily used for research purposes only.
Overall, the preparation and manufacture of the halogens require specialized equipment and safety precautions due to the highly reactive and potentially hazardous nature of these elements.
Who is Required p-Block Elements Group 17 Preparation/Manufacture
The preparation and manufacture of Group 17 p-block elements, also known as halogens, are of interest to chemists, researchers, and industries that use these elements in various applications. Some of the industries that utilize halogens include pharmaceuticals, chemical production, electronics, water treatment, and agriculture. The properties of halogens, such as their high reactivity, ability to form strong oxidizing agents, and affinity for electrons, make them useful in a wide range of applications. For example, chlorine is used as a disinfectant in water treatment, bromine is used as a flame retardant in plastics, and iodine is used in pharmaceuticals as an antiseptic and disinfectant. Understanding the preparation and manufacture of halogens is essential for the safe and efficient use of these elements in various industries.
When is Required p-Block Elements Group 17 Preparation/Manufacture
The preparation and manufacture of p-block elements in Group 17, also known as halogens, is required whenever these elements are needed for various applications, such as in the production of chemicals, pharmaceuticals, and electronics, or in water treatment and agriculture. The demand for halogens varies depending on the specific application, and their production is ongoing to meet these needs.
For example, the demand for chlorine in water treatment and industrial processes is high, and its production is ongoing to meet these needs. Similarly, the demand for bromine in the production of flame retardants for plastics and textiles is also high. Iodine is used in the pharmaceutical industry as an antiseptic and disinfectant and is therefore in demand for the production of medicines. The demand for astatine is limited due to its scarcity and high radioactivity, and it is primarily used for research purposes only.
The ongoing research and development of new applications for halogens also drive the need for their preparation and manufacture. Overall, the production of p-block elements in Group 17 is a continuous process that occurs whenever there is a demand for these elements in various industries and applications.
Where is Required p-Block Elements Group 17 Preparation/Manufacture
The preparation and manufacture of p-block elements in Group 17, also known as halogens, occurs in various locations worldwide, depending on the specific element and application.
For example, the production of chlorine, which is used extensively in the chemical industry and for water treatment, takes place in many countries, including the United States, China, and India. Bromine is mainly produced in the United States and Israel, which are the largest producers of this element. Iodine production is also widespread, with Chile, Japan, and the United States being major producers.
The preparation of astatine is much more limited due to its scarcity and high radioactivity. Astatine is primarily produced in research laboratories through nuclear reactions, and its use is limited to scientific research.
Overall, the production of halogens is a global process that occurs in various locations worldwide, depending on the specific element and the demand for it in various applications. The ongoing research and development of new applications for halogens also drive their production and manufacture in different parts of the world.
How is Required p-Block Elements Group 17 Preparation/Manufacture
The preparation and manufacture of p-block elements in Group 17, also known as halogens, involve different methods and processes depending on the specific element. Here are some of the common methods used for the preparation and manufacture of halogens:
- Fluorine: Fluorine is usually produced by the electrolysis of a solution of hydrogen fluoride and potassium fluoride in a metal container. The electrolysis process is carried out at a low temperature and high pressure to prevent the explosive reaction of fluorine gas with other substances.
- Chlorine: Chlorine is typically produced by the electrolysis of a solution of sodium chloride (brine) in a diaphragm cell. Chlorine gas is collected at the anode, while hydrogen gas is collected at the cathode.
- Bromine: Bromine can be prepared by treating a solution of a bromide salt, such as potassium bromide, with chlorine gas. The chlorine oxidizes the bromide ion to bromine, which is then collected by distillation.
- Iodine: Iodine is typically prepared by the reaction of iodide salts, such as potassium iodide, with oxidizing agents such as hydrogen peroxide or chlorine. The iodine is liberated as a solid, which is then collected by filtration and sublimation.
- Astatine: Astatine is a highly radioactive element, and its preparation is challenging due to its scarcity and short half-life. Astatine can be produced by bombarding bismuth-209 with alpha particles in a cyclotron or nuclear reactor.
Overall, the preparation and manufacture of halogens require specialized equipment and safety precautions due to the highly reactive and potentially hazardous nature of these elements. The processes involved in their production are complex and require careful control of temperature, pressure, and reactant concentrations to ensure the desired outcome.
Case Study on p-Block Elements Group 17 Preparation/Manufacture
Case Study: Preparation and Manufacture of Chlorine
Chlorine is a p-block element in Group 17 and is widely used in various industries, including water treatment, chemical production, and paper manufacturing. The preparation and manufacture of chlorine involve a complex process that requires specialized equipment and safety precautions to ensure worker safety and product quality.
One of the most common methods for the production of chlorine is the electrolysis of brine (sodium chloride solution). The electrolysis process involves passing an electric current through a solution of brine in an electrolytic cell, which causes the chloride ions to oxidize to form chlorine gas at the anode, and hydrogen gas to form at the cathode.
The process of chlorine production involves the following steps:
- Preparation of brine solution: The brine solution is typically prepared by dissolving rock salt (sodium chloride) in water. The solution is then purified to remove impurities such as calcium and magnesium ions, which can interfere with the electrolysis process.
- Electrolysis of brine: The brine solution is fed into an electrolytic cell, which consists of a series of anodes and cathodes separated by a diaphragm or membrane. The cell is filled with brine solution, and an electric current is passed through the cell. Chlorine gas is produced at the anode, and hydrogen gas is produced at the cathode. The diaphragm or membrane helps to prevent the mixing of the two gases.
- Separation of chlorine gas: The chlorine gas produced at the anode is collected and purified by passing it through a series of scrubbers and filters. The scrubbers remove impurities such as sulfur dioxide and hydrogen chloride, which can react with the chlorine gas to form unwanted byproducts. The filters remove any remaining impurities and water vapor.
- Compression and storage: The purified chlorine gas is compressed into cylinders or tankers and stored under pressure until it is ready for use. Chlorine gas is typically stored in a cool, dry place away from sunlight and sources of heat.
The production of chlorine is a continuous process that requires careful control of the electrolysis conditions, such as temperature, current density, and brine concentration, to ensure high-quality product output. Safety measures must also be taken to protect workers from exposure to chlorine gas, which can be toxic and corrosive at high concentrations.
In conclusion, the production of chlorine involves a complex and sophisticated process that requires specialized equipment, safety precautions, and careful attention to process parameters to ensure the efficient and safe manufacture of this essential p-block element.
White paper on p-Block Elements Group 17 Preparation/Manufacture
White Paper: Preparation and Manufacture of p-Block Elements in Group 17
Introduction:
The p-block elements in Group 17, commonly known as halogens, are a vital part of the chemical industry. They are widely used in various applications, including water treatment, chemical production, pharmaceuticals, and many others. The preparation and manufacture of these elements require specialized processes that involve careful control of temperature, pressure, and reactant concentrations to ensure high-quality product output.
This white paper provides an overview of the preparation and manufacture of p-block elements in Group 17, with a focus on the most commonly used elements, namely fluorine, chlorine, bromine, and iodine.
Fluorine:
Fluorine is the most reactive of all the halogens and is notoriously difficult to prepare and handle due to its highly reactive nature. Fluorine is typically produced by the electrolysis of a solution of hydrogen fluoride and potassium fluoride in a metal container. The electrolysis process is carried out at a low temperature and high pressure to prevent the explosive reaction of fluorine gas with other substances. Fluorine is a highly reactive gas that is used in the production of various chemicals, including refrigerants, solvents, and plastics.
Chlorine:
Chlorine is the most commonly produced halogen and is used in various applications, including water treatment, chemical production, and paper manufacturing. Chlorine is typically produced by the electrolysis of brine (sodium chloride solution) in an electrolytic cell. The electrolysis process involves passing an electric current through a solution of brine in an electrolytic cell, which causes the chloride ions to oxidize to form chlorine gas at the anode, and hydrogen gas to form at the cathode. The process of chlorine production involves several steps, including preparation of the brine solution, electrolysis of the brine, separation of chlorine gas, compression, and storage.
Bromine:
Bromine is a reddish-brown liquid that is used in various applications, including flame retardants, water treatment, and photography. Bromine can be prepared by treating a solution of a bromide salt, such as potassium bromide, with chlorine gas. The chlorine oxidizes the bromide ion to bromine, which is then collected by distillation. Bromine is a highly reactive and toxic liquid that must be handled with care to prevent exposure to humans and the environment.
Iodine:
Iodine is a solid halogen that is used in various applications, including medicine, photography, and analytical chemistry. Iodine is typically prepared by the reaction of iodide salts, such as potassium iodide, with oxidizing agents such as hydrogen peroxide or chlorine. The iodine is liberated as a solid, which is then collected by filtration and sublimation. Iodine is a relatively stable solid that can be stored and transported without special precautions.
Conclusion:
The preparation and manufacture of p-block elements in Group 17 are essential for the chemical industry. The methods and processes involved in their production are complex and require specialized equipment and safety precautions to ensure worker safety and product quality. The production of these elements must be carefully controlled to ensure high-quality product output and prevent environmental and health hazards.
