Group 17 of the periodic table, also known as the halogens, consists of the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Here are some of their general properties:
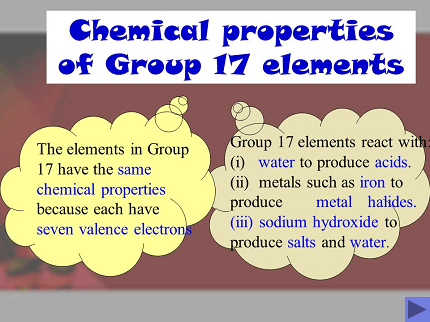
- Electronic configuration: Group 17 elements have seven valence electrons in their outermost shell, with the general configuration ns²np⁵.
- Atomic size: The atomic radius of the halogens increases down the group due to an increase in the number of electron shells.
- Electronegativity: Halogens are highly electronegative, meaning they have a strong attraction for electrons in chemical bonds. Electronegativity increases up the group due to the decrease in atomic radius.
- Melting and boiling points: The halogens have low melting and boiling points compared to other elements due to weak intermolecular forces of attraction between their molecules.
- Oxidation states: Halogens can exhibit a range of oxidation states, with the most common being -1. Fluorine is the most electronegative element, and can even form compounds with a positive oxidation state.
- Reactivity: Halogens are highly reactive due to their electronegativity and tendency to gain electrons. They react with most metals to form salts, and with hydrogen to form hydrogen halides.
- Solubility: The solubility of the halogens in water decreases down the group due to an increase in molecular size and London dispersion forces.
- Color: Halogens exhibit characteristic colors in their elemental form. Fluorine is pale yellow, chlorine is green-yellow, bromine is red-brown, iodine is violet, and astatine is black.
Overall, the halogens are a highly reactive group of elements with a variety of chemical properties.
What is Required p-Block Elements Group 17 Properties
The p-block elements in Group 17 of the periodic table are called the halogens. They include the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Here are some of the required properties of these elements:
- Electronic configuration: Group 17 elements have seven valence electrons in their outermost shell, with the general configuration ns²np⁵.
- Atomic radius: The atomic radius of halogens increases down the group due to an increase in the number of electron shells.
- Electronegativity: Halogens are highly electronegative elements, meaning they have a strong attraction for electrons in chemical bonds. Electronegativity increases up the group due to the decrease in atomic radius.
- Ionization energy: The ionization energy of halogens increases up the group due to the decrease in atomic size and the increase in nuclear charge.
- Reactivity: Halogens are highly reactive elements due to their electronegativity and tendency to gain electrons. They react with most metals to form salts, and with hydrogen to form hydrogen halides. Reactivity decreases down the group due to the increase in atomic size.
- Physical state: The halogens are diatomic molecules in their elemental state at room temperature and pressure, with F2 and Cl2 being gases, Br2 a liquid, and I2 a solid.
- Melting and boiling points: The halogens have low melting and boiling points compared to other elements due to weak intermolecular forces of attraction between their molecules.
- Oxidation states: Halogens can exhibit a range of oxidation states, with the most common being -1. Fluorine is the most electronegative element, and can even form compounds with a positive oxidation state.
- Solubility: The solubility of the halogens in water decreases down the group due to an increase in molecular size and London dispersion forces.
Overall, the halogens are a highly reactive group of elements with a variety of chemical properties.
When is Required p-Block Elements Group 17 Properties
I’m still not entirely sure what you are asking, but if you’re asking when knowledge of the properties of p-block elements in Group 17 is required, that would depend on the context.
If you’re a student of chemistry, you would likely learn about the properties of the halogens (Group 17 elements) as part of your curriculum in a high school or university level course. Understanding these properties would be required to comprehend topics related to chemical bonding, reactivity, and chemical reactions involving halogens.
If you’re a researcher or professional in a field related to chemistry, the knowledge of the properties of the halogens would be required when working on projects or experiments involving these elements.
In summary, the required knowledge of p-block elements in Group 17 and their properties would depend on the context and the level of education or profession involved.
Where is Required p-Block Elements Group 17 Properties
Again, I’m not entirely sure what you are asking for. If you are asking where p-block elements in Group 17 can be found, they are located in the p-block of the periodic table. Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) are the five elements that belong to this group.
If you are asking where knowledge of the properties of p-block elements in Group 17 is required, that would depend on the context, as I explained in my previous response. This knowledge could be required in a classroom or laboratory setting, in a research or professional environment, or in any other context where understanding of chemistry and chemical properties is necessary.
How is Required p-Block Elements Group 17 Properties
The properties of p-block elements in Group 17 are determined by a number of factors, including their electronic configurations, atomic and ionic radii, electronegativity, ionization energy, reactivity, and chemical bonding.
For example, the halogens (Group 17 elements) have a general electronic configuration of ns²np⁵, meaning they have seven valence electrons in their outermost shell. This configuration determines their reactivity and tendency to gain an electron to achieve a stable octet configuration. The size of the atoms and ions also plays a role in their properties, with halogens having relatively small atomic and ionic radii due to their high nuclear charge and effective nuclear charge.
The high electronegativity of the halogens results from their small size and strong nuclear charge, making them highly attractive to electrons in chemical bonding. This can lead to the formation of covalent and ionic compounds, such as hydrogen halides and metal halides. The reactivity of the halogens decreases down the group due to the increase in atomic size and the weaker attraction to electrons.
In summary, the properties of p-block elements in Group 17 are determined by a variety of factors related to their electronic configuration, atomic structure, and chemical behavior, and are essential to understanding their behavior and reactions in various contexts.
Production of p-Block Elements Group 17 Properties
The p-block elements in Group 17 of the periodic table, also known as halogens, are naturally occurring elements that can be found in the Earth’s crust and oceans.
The most abundant halogen is chlorine, which is typically produced by the electrolysis of brine (a solution of sodium chloride in water). This process involves passing an electric current through the brine, which causes the chloride ions to undergo oxidation at the anode, producing chlorine gas. Sodium hydroxide (NaOH) and hydrogen gas are also produced as byproducts of the reaction.
Fluorine is typically produced through the electrolysis of molten potassium fluoride (KF) and hydrogen fluoride (HF) in a process known as the “Hall-Heroult process.” This is a complex and expensive process that requires careful handling of the highly reactive and toxic fluorine gas.
Bromine and iodine are typically produced from seawater or brine through a series of chemical reactions. Bromine is extracted from seawater by treating it with chlorine gas to produce bromine and sodium chloride. Iodine is typically produced from seaweed or other organic sources through a process known as “iodine value extraction,” which involves burning the source material and extracting the iodine from the resulting ash.
Astatine, the rarest halogen, is typically produced in minute quantities through the decay of radioactive isotopes. It is not commonly produced in significant quantities for commercial use.
Case Study on p-Block Elements Group 17 Properties
One example of a case study involving the properties of p-block elements in Group 17 is the use of chlorine gas in water treatment.
Chlorine gas is a strong oxidizing agent and a powerful disinfectant that is commonly used to treat drinking water and wastewater to kill harmful bacteria and viruses. When chlorine gas is added to water, it reacts with organic compounds and other contaminants to form hypochlorous acid (HOCl) and hypochlorite ions (OCl-), which are highly reactive and can destroy microorganisms.
However, the use of chlorine gas in water treatment has some potential drawbacks. Chlorine gas can be toxic if inhaled, and exposure to high levels can cause respiratory problems and other health issues. Additionally, the reaction of chlorine with organic compounds can produce potentially harmful byproducts, such as trihalomethanes (THMs) and haloacetic acids (HAAs), which are known carcinogens.
To address these concerns, alternative disinfection methods have been developed, such as ultraviolet (UV) radiation and ozonation. UV radiation uses high-energy light to destroy microorganisms, while ozonation involves the use of ozone gas to oxidize and disinfect water. These methods can be more expensive and technically challenging than traditional chlorine disinfection, but they offer benefits such as reduced production of harmful disinfection byproducts.
In this case study, the properties of chlorine as a halogen with strong oxidizing and disinfecting properties are central to its use in water treatment. However, the potential health risks and production of harmful byproducts associated with chlorine disinfection highlight the importance of understanding and managing the properties of p-block elements in Group 17 in order to ensure safe and effective use in various applications.
White paper on p-Block Elements Group 17 Properties
Introduction:
The p-block elements in Group 17 of the periodic table, also known as halogens, are essential components of many important chemical compounds and materials. These elements have unique properties that make them useful in a variety of applications, from water treatment to the production of electronic devices.
Properties:
The halogens are characterized by their electronic configuration of ns²np⁵, which gives them seven valence electrons in their outermost shell. This configuration makes them highly reactive and likely to gain an electron to achieve a stable octet configuration. As a result, the halogens are strong oxidizing agents and are capable of forming a wide range of chemical compounds with other elements.
The size of the halogen atoms and ions is relatively small due to their high nuclear charge and effective nuclear charge. This small size results in high electronegativity, meaning that halogens have a strong attraction to electrons in chemical bonding. The reactivity of the halogens decreases down the group due to the increase in atomic size and the weaker attraction to electrons.
The halogens are also known for their ability to form hydrogen halides (HF, HCl, HBr, and HI) with hydrogen, which are important in a range of industrial applications. They can also form metal halides, such as sodium chloride (NaCl), which is essential to life and is used in a wide range of applications.
Applications:
The properties of the halogens make them useful in a wide range of applications. Chlorine, for example, is commonly used as a disinfectant in water treatment to kill harmful bacteria and viruses. Bromine is used in the production of flame retardants, and iodine is used in the production of pharmaceuticals and dyes. Fluorine is used in the production of refrigerants and electronic devices.
The unique properties of the halogens also make them useful in the production of many important chemical compounds. For example, hydrogen chloride gas (HCl) is used in the production of vinyl chloride, which is used to make PVC (polyvinyl chloride) plastic. Chlorine gas is used in the production of polyurethane foam, which is used in mattresses, cushions, and other products.
Conclusion:
The p-block elements in Group 17, or halogens, are a diverse and important group of elements with a wide range of properties and applications. Their high electronegativity, reactivity, and ability to form a wide range of chemical compounds make them useful in a range of industrial and commercial applications. However, their unique properties also require careful handling and management to ensure safe and effective use in various contexts. Understanding the properties of p-block elements in Group 17 is essential to advancing their applications and improving their utility in various fields.
