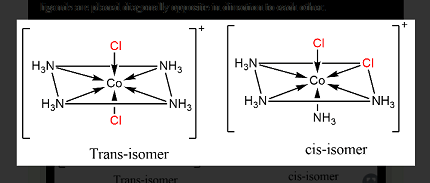
Cis-trans isomerism is a type of stereoisomerism that arises due to the restricted rotation around a double bond or in a cyclic compound. In cis-trans isomers, the relative positions of substituent groups or atoms differ around a double bond or in a cyclic structure. In other words, the cis isomer has substituent groups or atoms on the same side of the double bond or in the same orientation in a cyclic structure, while the trans isomer has substituent groups or atoms on opposite sides of the double bond or in different orientations in a cyclic structure. For example, in cis-2-butene, the two methyl groups are on the same side of the double bond, while in trans-2-butene, the two methyl groups are on opposite sides of the double bond.
Ionization isomerism is a type of structural isomerism that arises due to the interchange of ions between the ligands and the central metal ion in a coordination compound. In ionization isomers, the same ligands are present in both isomers, but the arrangement of the ligands around the central metal ion is different due to the exchange of anionic or cationic ligands. For example, in the coordination compound [Co(NH3)5Br]Cl2, the chloride ions can be exchanged with the bromide ions to form the ionization isomer [Co(NH3)5Br]Br2, where the bromide ions are coordinated to the central cobalt ion.
In summary, cis-trans isomerism arises due to the restricted rotation around a double bond or in a cyclic compound, while ionization isomerism arises due to the interchange of ions between the ligands and the central metal ion in a coordination compound.
What is Required Cis-trans and Ionization isomerism
Cis-trans isomerism requires a molecule with a double bond or a cyclic structure that restricts rotation around the ring. The substituent groups or atoms on each side of the double bond or within the cyclic structure must be different. For example, a molecule like 1,2-dichloroethene can exhibit cis-trans isomerism because the two chlorine atoms are on opposite sides of the double bond in the trans isomer, while they are on the same side in the cis isomer.
Ionization isomerism requires a coordination compound, which is a compound containing a central metal ion and surrounding ligands that can donate electrons to the metal ion. The ligands must be capable of exchanging ions with each other to create different ionic isomers. For example, a coordination compound like [Co(NH3)5Br]Cl2 can exhibit ionization isomerism because the chloride ions and bromide ions can exchange with each other, creating two different compounds:
[Co(NH3)5Br]Cl2 and [Co(NH3)5Cl]Br2.
In general, both cis-trans isomerism and ionization isomerism require specific molecular or structural features in order to occur.
When is Required Cis-trans and Ionization isomerism
Cis-trans isomerism is required when a molecule has a double bond or a cyclic structure that restricts rotation around the ring and has substituent groups or atoms on each side of the double bond or within the cyclic structure that are different. Examples of molecules that exhibit cis-trans isomerism include alkenes, such as butene, and cyclic compounds, such as cyclohexane.
Ionization isomerism is required when dealing with coordination compounds, which are compounds that contain a central metal ion surrounded by ligands that can donate electrons to the metal ion. In ionization isomerism, the same ligands are present in both isomers, but the arrangement of the ligands around the central metal ion is different due to the exchange of anionic or cationic ligands. Examples of coordination compounds that exhibit ionization isomerism include [Co(NH3)5Br]Cl2 and [Co(NH3)5Cl]Br2. Ionization isomerism is particularly important in the study of transition metal complexes, where it plays a critical role in their chemical and biological properties.
Where is Required Cis-trans and Ionization isomerism
Cis-trans isomerism can be found in molecules that contain a double bond or a cyclic structure, such as alkenes, cycloalkenes, and cyclic compounds. These molecules are commonly found in organic chemistry, and their stereochemistry (the arrangement of atoms or groups in space) is important in determining their properties and reactivity.
Ionization isomerism is observed in coordination compounds, which are commonly found in inorganic chemistry. Coordination compounds are widely used in fields such as catalysis, medicine, and materials science. Ionization isomerism arises due to the interchange of ions between the ligands and the central metal ion in a coordination compound, and it is important in determining the chemical and biological properties of these compounds. Coordination compounds are commonly found in nature, such as in the active sites of enzymes, and they have important applications in industry and medicine.
Production of Cis-trans and Ionization isomerism
Cis-trans isomers can be produced through a variety of synthetic methods in organic chemistry. For example, the stereoselective synthesis of alkenes using methods such as the Wittig reaction, the Horner-Wadsworth-Emmons reaction, and the Grubbs olefin metathesis reaction can lead to the formation of cis-trans isomers. Additionally, some cyclic compounds can be synthesized through ring-closing reactions, which can result in the formation of cis-trans isomers.
Ionization isomers can be produced by the interchange of ions between the ligands and the central metal ion in a coordination compound. The production of ionization isomers can be influenced by factors such as the nature of the ligands, the charge of the metal ion, and the solvent used. For example, changing the solvent in which a coordination compound is dissolved can result in the formation of different ionization isomers. The production of ionization isomers can also be influenced by changes in temperature, pressure, or pH, which can affect the equilibrium between the different ionic forms. In general, the production of cis-trans and ionization isomers requires careful control over reaction conditions and a detailed understanding of the chemical properties of the compounds involved.
How is Required Cis-trans and Ionization isomerism
Cis-trans isomerism arises from the restriction of rotation around a double bond or within a cyclic structure. In a molecule with a double bond, the substituent groups or atoms on each side of the bond can be arranged either on the same side of the bond (cis) or on opposite sides (trans). In a cyclic structure, the arrangement of the substituent groups or atoms around the ring can result in different isomers. The difference in the spatial arrangement of the atoms or groups gives rise to different physical and chemical properties of the isomers.
Ionization isomerism arises from the interchange of ions between the ligands and the central metal ion in a coordination compound. The isomers differ in the arrangement of the ligands around the central metal ion, and they have different physical and chemical properties. The interconversion between ionization isomers can be influenced by various factors, such as the nature of the ligands and the metal ion, the pH of the solution, and the temperature. The interchange of ions between the ligands and the metal ion is typically reversible and can be affected by changing the equilibrium conditions of the reaction.
In both cases, the isomerism arises from the differences in the spatial arrangement of the atoms or groups around the molecule or the central metal ion. These differences in spatial arrangement can have a significant impact on the physical and chemical properties of the isomers, such as their melting and boiling points, reactivity, and bioactivity.
Case Study on Cis-trans and Ionization isomerism
One example of the importance of cis-trans isomerism is in the synthesis of the drug thalidomide. Thalidomide was originally developed as a sedative and anti-nausea medication, but it was later found to cause severe birth defects in infants born to women who had taken the drug during pregnancy. The reason for this was found to be due to the presence of two different isomers of the drug: the R-thalidomide and the S-thalidomide isomers.
The R-thalidomide isomer has sedative and anti-nausea properties, while the S-thalidomide isomer is responsible for the birth defects. The reason for this is due to the fact that the S-thalidomide isomer can undergo racemization in the body, converting to the R-thalidomide isomer, which is active as a sedative. However, during this process, the S-thalidomide isomer can also form a reactive intermediate that can lead to the formation of DNA adducts, which can result in birth defects.
Another example of the importance of isomerism is in the study of coordination compounds. Coordination compounds are widely used in catalysis, and the properties of these compounds can be influenced by the arrangement of the ligands around the central metal ion. For example, the coordination compound cisplatin is used as a chemotherapeutic agent in cancer treatment. The efficacy of cisplatin is due to its ability to bind to DNA and prevent cell division, but this process can also lead to damage to healthy cells. Studies have shown that the effectiveness of cisplatin can be influenced by the arrangement of the ligands around the central platinum ion, with the cis isomer being more effective than the trans isomer.
In ionization isomerism, the properties of coordination compounds can also be influenced by the arrangement of the ligands around the central metal ion. For example, the ionization isomers [Co(NH3)5Cl]Br2 and [Co(NH3)5Br]Cl2 have the same ligands but different arrangements, and they have different physical and chemical properties. The interconversion between these isomers can be influenced by factors such as the pH of the solution and the temperature, and this interconversion can have an impact on the reactivity and bioactivity of the coordination compound.
In both cases, the study of isomerism is important for understanding the properties and behavior of molecules and coordination compounds, and for developing new drugs and materials with specific properties.
White paper on Cis-trans and Ionization isomerism
Introduction:
Isomers are compounds that have the same molecular formula but different arrangements of atoms or groups in space. Isomerism can arise in many different ways, including cis-trans isomerism and ionization isomerism. Cis-trans isomerism arises from the restriction of rotation around a double bond or within a cyclic structure, while ionization isomerism arises from the interchange of ions between the ligands and the central metal ion in a coordination compound. Understanding isomerism is important for understanding the properties and behavior of molecules and coordination compounds and for developing new drugs and materials with specific properties.
Cis-Trans Isomerism:
Cis-trans isomerism arises from the restriction of rotation around a double bond or within a cyclic structure. In a molecule with a double bond, the substituent groups or atoms on each side of the bond can be arranged either on the same side of the bond (cis) or on opposite sides (trans). In a cyclic structure, the arrangement of the substituent groups or atoms around the ring can result in different isomers. The difference in the spatial arrangement of the atoms or groups gives rise to different physical and chemical properties of the isomers.
One example of the importance of cis-trans isomerism is in the synthesis of the drug thalidomide. Thalidomide was originally developed as a sedative and anti-nausea medication, but it was later found to cause severe birth defects in infants born to women who had taken the drug during pregnancy. The reason for this was found to be due to the presence of two different isomers of the drug: the R-thalidomide and the S-thalidomide isomers.
Ionization Isomerism:
Ionization isomerism arises from the interchange of ions between the ligands and the central metal ion in a coordination compound. The isomers differ in the arrangement of the ligands around the central metal ion, and they have different physical and chemical properties. The interconversion between ionization isomers can be influenced by various factors, such as the nature of the ligands and the metal ion, the pH of the solution, and the temperature. The interchange of ions between the ligands and the metal ion is typically reversible and can be affected by changing the equilibrium conditions of the reaction.
In both cases, the isomerism arises from the differences in the spatial arrangement of the atoms or groups around the molecule or the central metal ion. These differences in spatial arrangement can have a significant impact on the physical and chemical properties of the isomers, such as their melting and boiling points, reactivity, and bioactivity.
Applications:
Cis-trans isomerism has a wide range of applications, particularly in the pharmaceutical industry. The isomers can have different pharmacological properties, which can be exploited to develop drugs with specific activities. For example, the drug thalidomide has different isomers with different activities, as discussed earlier.
Ionization isomerism is important in the study of coordination compounds, which are widely used in catalysis, and the properties of these compounds can be influenced by the arrangement of the ligands around the central metal ion. Understanding ionization isomerism is crucial in the development of new catalysts and other materials with specific properties.
Conclusion:
In conclusion, isomerism is an important concept in chemistry that arises from the differences in spatial arrangement of atoms or groups around molecules or central metal ions. Cis-trans isomerism and ionization isomerism are two examples of isomerism that have important applications in pharmaceuticals, materials science, and other areas. Understanding isomerism is crucial for developing new drugs and materials with specific properties and for understanding the behavior of molecules and coordination compounds.
