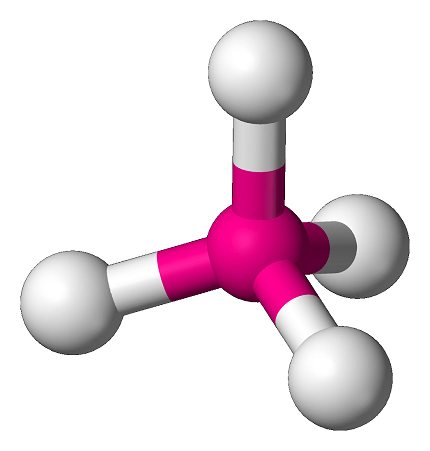
The term “tetrahedral” can refer to several different concepts in mathematics and geometry, but most commonly it refers to a specific type of geometric shape. A tetrahedron is a three-dimensional shape with four triangular faces, six edges, and four vertices.
The tetrahedron is the simplest of all the regular polyhedra, which are three-dimensional shapes made up of identical regular polygons. Each of the four faces of a tetrahedron is an equilateral triangle, and all four vertices are located at the same distance from each other, forming what is known as a regular tetrahedron.
In chemistry, the term tetrahedral is used to describe the arrangement of atoms in certain molecules. For example, the methane molecule (CH4) has a tetrahedral shape, with the carbon atom at the center and four hydrogen atoms arranged symmetrically around it. This tetrahedral shape is important in understanding the molecular geometry and properties of many different types of molecules.
What is Required Coordination Compounds Tetrahedral
Tetrahedral coordination compounds are a type of coordination complex in which the central metal ion is surrounded by four ligands, arranged in a tetrahedral shape. In order for a coordination compound to have a tetrahedral geometry, it must meet certain requirements:
- The central metal ion must have a coordination number of 4. This means that it can form four chemical bonds with ligands.
- The ligands must be small and of similar size. If the ligands are too large, they may interfere with each other and prevent a tetrahedral arrangement.
- The ligands must be of the same type, or at least have similar properties. This ensures that they will bond to the central metal ion in a similar way.
- The ligands must have a high degree of symmetry. This allows them to arrange themselves around the central metal ion in a tetrahedral shape.
Examples of coordination compounds that can have a tetrahedral geometry include tetrachloromethane (CCl4), which has a carbon atom at its center surrounded by four chlorine atoms, and the cobalt(II) complex [CoCl4]2-, which has a cobalt ion at its center surrounded by four chloride ions.
Who is Required Coordination Compounds Tetrahedral
The concept of tetrahedral coordination compounds is a fundamental concept in coordination chemistry, a field of chemistry that focuses on the study of metal ions and the molecules or ions that surround them.
The understanding of tetrahedral coordination compounds is important in many areas of chemistry, including biochemistry, materials science, and catalysis. For example, many enzymes and proteins contain metal ions surrounded by ligands in a tetrahedral arrangement, which is critical to their function.
In addition, the study of tetrahedral coordination compounds has practical applications in the development of new materials with specific properties, such as improved strength or magnetic properties. Tetrahedral coordination compounds are also used as catalysts in a wide range of chemical reactions, including the synthesis of pharmaceuticals and other important compounds.
When is Required Coordination Compounds Tetrahedral
Tetrahedral coordination compounds are required when a metal ion is surrounded by four ligands that bond to it in a tetrahedral arrangement. This type of coordination geometry is commonly observed in coordination complexes of transition metals, where the metal ion typically has a coordination number of four.
Tetrahedral coordination compounds are important in many areas of chemistry, including biochemistry, materials science, and catalysis. For example, in biochemistry, many metalloproteins contain metal ions surrounded by four ligands in a tetrahedral arrangement, which is critical to their function. In materials science, tetrahedral coordination compounds are used to develop new materials with specific properties, such as improved strength or magnetic properties. In catalysis, tetrahedral coordination compounds are often used as catalysts in a wide range of chemical reactions, including the synthesis of pharmaceuticals and other important compounds.
Overall, tetrahedral coordination compounds are important in understanding the properties and behavior of a wide range of chemical systems, and their study has practical applications in many different fields of chemistry.
Where is Required Coordination Compounds Tetrahedral
Tetrahedral coordination compounds can be found in a variety of chemical systems and environments. In general, they are most commonly observed in coordination complexes of transition metals, which are metal ions that can form a variety of different coordination geometries due to their partially filled d-orbitals.
Tetrahedral coordination compounds can be found in many different types of materials, including metalloproteins, minerals, and synthetic compounds. They are also commonly used as catalysts in a wide range of chemical reactions, including the synthesis of pharmaceuticals and other important compounds.
In biological systems, tetrahedral coordination compounds are often found in metalloproteins and enzymes, where they play important roles in catalyzing reactions and regulating biological processes. For example, the heme group in hemoglobin, which is responsible for binding oxygen in the blood, contains an iron ion coordinated by four nitrogen atoms in a tetrahedral arrangement.
In materials science, tetrahedral coordination compounds are used to develop new materials with specific properties, such as improved strength or magnetic properties. They are also used as building blocks for supramolecular structures and other complex materials.
Overall, tetrahedral coordination compounds are important in a wide range of chemical systems and environments, and their study has practical applications in many different fields of chemistry.
How is Required Coordination Compounds Tetrahedral
Tetrahedral coordination compounds are formed when a metal ion is surrounded by four ligands that bond to it in a tetrahedral arrangement. The formation of a tetrahedral coordination complex involves the following steps:
- The metal ion attracts the ligands towards itself through electrostatic forces.
- The ligands approach the metal ion and form coordinate covalent bonds with it. In this type of bond, one atom (in this case, the metal ion) donates a pair of electrons to the other atom (the ligand), forming a bond.
- The ligands arrange themselves in a tetrahedral shape around the metal ion. This arrangement is determined by the size and shape of the ligands, as well as their electronic properties.
- The resulting tetrahedral coordination complex is stabilized by various types of intermolecular interactions, such as hydrogen bonding, Van der Waals forces, and electrostatic interactions.
The geometry of tetrahedral coordination complexes can be analyzed using a variety of techniques, including X-ray crystallography, NMR spectroscopy, and computational modeling. These techniques can provide information about the bond lengths, bond angles, and other structural parameters of the complex, which can be used to understand its properties and behavior.
Overall, the formation and study of tetrahedral coordination compounds is an important area of chemistry that has broad applications in many different fields.
Case Study on Coordination Compounds Tetrahedral
One example of a tetrahedral coordination compound is tetrakis(dimethylamido) titanium(IV), or Ti(NMe2)4. This compound is used as a precursor for the synthesis of titanium-containing materials, such as thin films for electronics and catalytic materials for chemical reactions.
Ti(NMe2)4 is a tetrahedral coordination complex, with the titanium ion coordinated by four dimethylamide ligands in a tetrahedral arrangement. The synthesis of Ti(NMe2)4 typically involves the reaction of titanium tetrachloride (TiCl4) with dimethylamine (NMe2H) in the presence of a reducing agent such as lithium aluminum hydride (LiAlH4):
TiCl4 + 4 NMe2H + LiAlH4 → Ti(NMe2)4 + LiCl + AlCl3 + 2 H2
The resulting product is a white, crystalline solid that is soluble in a variety of organic solvents.
The tetrahedral coordination geometry of Ti(NMe2)4 is important for its properties and behavior. For example, the tetrahedral arrangement of the ligands around the titanium ion leads to a high degree of symmetry in the molecule, which can affect its reactivity and stability. In addition, the presence of the dimethylamide ligands can influence the electronic properties of the titanium ion, making it more or less reactive towards other molecules or ions.
Ti(NMe2)4 is commonly used as a precursor for the synthesis of titanium-containing materials, such as thin films for electronics and catalytic materials for chemical reactions. The tetrahedral coordination geometry of Ti(NMe2)4 can be used to control the structure and properties of these materials, making them more efficient or effective for their intended applications.
Overall, the study of tetrahedral coordination compounds such as Ti(NMe2)4 is important for the development of new materials with specific properties and applications in a variety of fields, including electronics, catalysis, and materials science.
White paper on Coordination Compounds Tetrahedral
Introduction
Coordination compounds are molecules or ions that consist of a central metal ion surrounded by ligands that form coordinate covalent bonds with the metal. The geometry of these complexes is determined by the arrangement of the ligands around the metal ion. One common coordination geometry is tetrahedral, in which the ligands are arranged in a tetrahedral shape around the metal ion. This white paper will provide an overview of tetrahedral coordination compounds, including their properties, synthesis, and applications.
Properties of Tetrahedral Coordination Compounds
Tetrahedral coordination compounds have a number of important properties that make them useful for a variety of applications. One of the most important properties is their symmetry, which is characterized by the presence of four ligands arranged in a tetrahedral shape around the metal ion. This symmetry can influence the reactivity and stability of the compound, as well as its electronic properties.
Another important property of tetrahedral coordination compounds is their ability to act as building blocks for more complex structures. By combining multiple tetrahedral coordination complexes together, it is possible to create larger, more complex structures with unique properties and functions.
Synthesis of Tetrahedral Coordination Compounds
Tetrahedral coordination compounds can be synthesized using a variety of methods, depending on the specific metal and ligands involved. One common method involves the reaction of a metal salt with a ligand in the presence of a base or acid. For example, the reaction of titanium tetrachloride with dimethylamine in the presence of lithium aluminum hydride can be used to synthesize tetrakis(dimethylamido) titanium(IV), which is a tetrahedral coordination complex.
Other methods for synthesizing tetrahedral coordination complexes include the use of templates or pre-formed ligand structures, which can guide the formation of the complex and control its geometry and properties.
Applications of Tetrahedral Coordination Compounds
Tetrahedral coordination compounds have a wide range of applications in many different fields, including materials science, catalysis, and biochemistry. In materials science, tetrahedral coordination complexes can be used as building blocks for the synthesis of new materials with specific properties, such as strength or magnetic properties.
In catalysis, tetrahedral coordination complexes can be used as catalysts for a wide range of chemical reactions, including the synthesis of pharmaceuticals and other important compounds. The tetrahedral geometry of the ligands around the metal ion can influence the reactivity and selectivity of the catalyst, making it more or less effective for a given reaction.
In biochemistry, tetrahedral coordination compounds are often found in metalloproteins and enzymes, where they play important roles in catalyzing reactions and regulating biological processes. For example, the heme group in hemoglobin, which is responsible for binding oxygen in the blood, contains an iron ion coordinated by four nitrogen atoms in a tetrahedral arrangement.
Conclusion
In conclusion, tetrahedral coordination compounds are an important class of molecules that have many applications in chemistry, materials science, catalysis, and biochemistry. Their unique properties and geometry make them useful for a wide range of applications, and their synthesis and study is an important area of research in chemistry and related fields.
