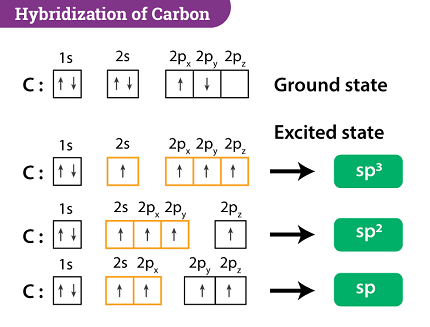
Carbon can undergo hybridization to form hybrid orbitals that can participate in chemical bonding. Hybridization of carbon involves the mixing of its valence orbitals, which are the 2s and three 2p orbitals, to form new hybrid orbitals. The most common hybridizations of carbon are sp, sp2, and sp3 hybridizations.
- sp hybridization: In sp hybridization, the carbon atom forms two hybrid orbitals by mixing one s orbital and one p orbital. These hybrid orbitals are linear and are oriented in opposite directions from each other. An example of a molecule that contains sp hybridized carbon is acetylene (C2H2).
- sp2 hybridization: In sp2 hybridization, the carbon atom forms three hybrid orbitals by mixing one s orbital and two p orbitals. These hybrid orbitals are arranged in a trigonal planar geometry and are oriented 120 degrees apart from each other. An example of a molecule that contains sp2 hybridized carbon is ethylene (C2H4).
- sp3 hybridization: In sp3 hybridization, the carbon atom forms four hybrid orbitals by mixing one s orbital and three p orbitals. These hybrid orbitals are arranged in a tetrahedral geometry and are oriented 109.5 degrees apart from each other. An example of a molecule that contains sp3 hybridized carbon is methane (CH4).
The type of hybridization that carbon undergoes depends on the number and types of atoms that it is bonded to in a molecule.
What is Required Basic Principles of Organic Chemistry Hybridisation of carbon
To understand the hybridization of carbon in organic chemistry, there are several basic principles that are required:
- Valence bond theory: This theory explains how atoms combine to form chemical bonds. According to this theory, atoms form bonds by overlapping their valence orbitals. In the case of carbon, it forms four covalent bonds by overlapping its hybrid orbitals with the hybrid or atomic orbitals of other atoms.
- Hybridization: Hybridization is the mixing of atomic orbitals to form hybrid orbitals. In organic chemistry, carbon can undergo sp, sp2, or sp3 hybridization, depending on the number of bonds it forms in a molecule.
- Geometry of hybrid orbitals: The geometry of the hybrid orbitals formed by carbon determines the shape of the molecule. For example, if carbon undergoes sp3 hybridization and forms four single bonds, the molecule has a tetrahedral shape. If carbon undergoes sp2 hybridization and forms three single bonds and a double bond, the molecule has a trigonal planar shape.
- Electronegativity: Electronegativity is the tendency of an atom to attract electrons towards itself in a chemical bond. In organic chemistry, the electronegativity of atoms bonded to carbon affects the polarity of the molecule.
Understanding these basic principles of organic chemistry is essential to explain the hybridization of carbon and its role in chemical bonding in organic molecules.
When is Required Basic Principles of Organic Chemistry Hybridisation of carbon
The required basic principles of organic chemistry include the concept of hybridization of carbon atoms, which is an essential topic in understanding the properties and reactions of organic molecules.
Hybridization of carbon refers to the process by which the orbitals of a carbon atom are reorganized to form hybrid orbitals that are suitable for bonding with other atoms. This allows carbon to form a variety of different types of bonds, including single, double, and triple bonds, and to adopt a range of different geometries.
The concept of hybridization of carbon was first introduced by Linus Pauling in the 1930s and has since become a fundamental principle in organic chemistry. It is typically covered in introductory organic chemistry courses at the undergraduate level and is a prerequisite for more advanced topics in the field.
So, if you are studying organic chemistry, you can expect to learn about the hybridization of carbon atoms early on in your coursework.
Where is Required Basic Principles of Organic Chemistry Hybridisation of carbon
The required basic principles of organic chemistry, including the concept of hybridization of carbon, are typically covered in organic chemistry courses offered by universities and colleges.
These courses are usually available at both the undergraduate and graduate level, and may be required as part of a chemistry or biochemistry major.
You may also find resources and tutorials on hybridization of carbon and other basic principles of organic chemistry online, through educational websites or chemistry textbooks. Additionally, there are several online platforms that offer courses and tutorials on organic chemistry, which may be helpful in supplementing your understanding of the subject.
How is Required Basic Principles of Organic Chemistry Hybridisation of carbon
The concept of hybridization of carbon atoms in organic chemistry is based on the idea that carbon can form four covalent bonds by combining its 2s and three 2p atomic orbitals.
To explain the observed properties and bonding behavior of organic molecules, it is necessary to invoke the concept of hybridization, which involves the reorganization of these orbitals to form hybrid orbitals that are better suited for bonding.
In particular, carbon atoms can undergo sp3 hybridization, where one 2s and three 2p orbitals combine to form four sp3 hybrid orbitals that are arranged in a tetrahedral geometry. This allows carbon to form single bonds with four other atoms, such as hydrogen or other carbon atoms.
Alternatively, carbon atoms can undergo sp2 hybridization, where one 2s and two 2p orbitals combine to form three sp2 hybrid orbitals and one unhybridized 2p orbital. This results in a trigonal planar geometry that allows carbon to form three sigma bonds and one pi bond, such as in the case of double bonds in organic molecules.
Finally, carbon atoms can undergo sp hybridization, where one 2s and one 2p orbital combine to form two sp hybrid orbitals and two unhybridized 2p orbitals. This leads to a linear geometry that allows carbon to form two sigma bonds and two pi bonds, such as in the case of triple bonds in organic molecules.
Overall, the concept of hybridization of carbon is a fundamental principle in understanding the bonding and reactivity of organic molecules and is an essential topic in organic chemistry.
Nomenclature of Basic Principles of Organic Chemistry Hybridisation of carbon
The nomenclature of organic compounds is a system used to give names to organic molecules based on their structure and composition.
In terms of the hybridization of carbon, the naming of organic compounds typically follows the rules outlined by the International Union of Pure and Applied Chemistry (IUPAC).
For example, organic compounds containing sp3-hybridized carbons are typically named as alkanes, with the suffix “-ane” indicating a single bond between carbons. The prefix indicates the number of carbons in the molecule, such as “meth-” for one carbon, “eth-” for two carbons, “prop-” for three carbons, and so on.
Organic compounds containing sp2-hybridized carbons are typically named as alkenes, with the suffix “-ene” indicating a double bond between carbons. The numbering of the carbons in the double bond is indicated by the lowest number on either side of the double bond.
Organic compounds containing sp-hybridized carbons are typically named as alkynes, with the suffix “-yne” indicating a triple bond between carbons. The numbering of the carbons in the triple bond is indicated by the lowest number on either side of the triple bond.
Other functional groups in organic molecules are also named according to specific rules outlined by IUPAC, such as alcohols, amines, carboxylic acids, and esters.
Overall, the nomenclature of organic compounds is an essential tool in communicating the structure and composition of organic molecules and is an important aspect of the study of organic chemistry.
Case Study on Basic Principles of Organic Chemistry Hybridisation of carbon
One potential case study on the basic principles of organic chemistry and the concept of hybridization of carbon could involve the synthesis of a common organic compound such as ethanol.
Ethanol is a simple organic compound composed of two carbon atoms, six hydrogen atoms, and one oxygen atom. Its chemical formula is C2H5OH, and it is commonly used as a fuel, solvent, and in the production of alcoholic beverages.
The synthesis of ethanol involves the combination of two molecules of a simpler organic compound, acetaldehyde (C2H4O), through a reaction known as a condensation reaction. In this reaction, the carbonyl group of one acetaldehyde molecule undergoes nucleophilic attack by the lone pair of electrons on the oxygen atom of a second acetaldehyde molecule, resulting in the formation of a new carbon-oxygen bond and the release of a water molecule.
The resulting product, ethanol, contains two sp3-hybridized carbon atoms, each with four sigma bonds. The carbon atoms in ethanol are arranged in a linear geometry, with the oxygen atom bonded to one of the carbons via a single bond and a hydrogen atom bonded to each carbon via a single bond.
Overall, the synthesis of ethanol illustrates the concept of hybridization of carbon and the importance of understanding the structure and bonding behavior of organic molecules in organic chemistry.
White paper on Basic Principles of Organic Chemistry Hybridisation of carbon
Here is a brief white paper on the basic principles of organic chemistry and the concept of hybridization of carbon.
Introduction
Organic chemistry is the study of the chemistry of carbon-based compounds, which are the building blocks of life and essential to many aspects of modern technology. The concept of hybridization of carbon is a fundamental principle in organic chemistry, which involves the reorganization of carbon atomic orbitals to form hybrid orbitals that are better suited for bonding. This white paper provides an overview of the basic principles of organic chemistry and the concept of hybridization of carbon.
Basic Principles of Organic Chemistry
The basic principles of organic chemistry involve the understanding of the structure, properties, and reactivity of carbon-based compounds. Organic molecules can be classified according to their functional groups, which are specific groups of atoms that confer unique properties and reactivity to the molecule. Some common functional groups include alcohols, amines, carboxylic acids, and esters.
The bonding behavior of organic molecules is based on the concept of valence bond theory, which describes how atoms form covalent bonds by sharing electrons. In organic molecules, carbon typically forms four covalent bonds by combining its 2s and three 2p atomic orbitals to form sp3 hybrid orbitals that are arranged in a tetrahedral geometry.
Concept of Hybridization of Carbon
The concept of hybridization of carbon involves the reorganization of carbon atomic orbitals to form hybrid orbitals that are better suited for bonding. Hybridization allows carbon to form single, double, and triple bonds with other atoms, and is the basis for the observed properties and bonding behavior of organic molecules.
Carbon can undergo three types of hybridization: sp3 hybridization, sp2 hybridization, and sp hybridization. Sp3 hybridization involves the combination of one 2s and three 2p atomic orbitals to form four sp3 hybrid orbitals, which are arranged in a tetrahedral geometry. Sp2 hybridization involves the combination of one 2s and two 2p atomic orbitals to form three sp2 hybrid orbitals and one unhybridized 2p orbital, which results in a trigonal planar geometry. Sp hybridization involves the combination of one 2s and one 2p atomic orbitals to form two sp hybrid orbitals and two unhybridized 2p orbitals, which results in a linear geometry.
Conclusion
The concept of hybridization of carbon is a fundamental principle in organic chemistry, which underlies the observed properties and bonding behavior of organic molecules. Understanding the structure and bonding behavior of organic molecules is essential for many areas of science and technology, including medicine, materials science, and energy. The principles of organic chemistry and the concept of hybridization of carbon provide a framework for the study and application of carbon-based compounds.
