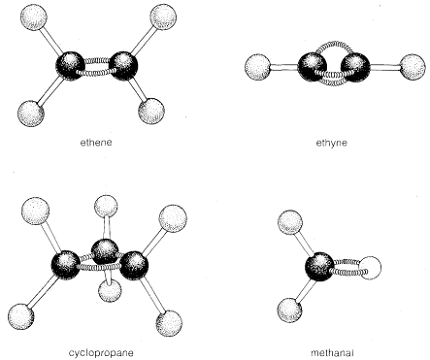
Simple organic molecules can have a variety of shapes depending on the arrangement of their atoms and the type of bonds between them. Here are some common shapes of simple organic molecules:
- Linear: A linear molecule has all atoms in a straight line. Examples include carbon dioxide (CO2) and acetylene (C2H2).
- Trigonal planar: A trigonal planar molecule has a central atom with three surrounding atoms arranged in a flat triangle. Examples include formaldehyde (H2CO) and boron trifluoride (BF3).
- Tetrahedral: A tetrahedral molecule has a central atom with four surrounding atoms arranged in a three-dimensional shape like a pyramid. Examples include methane (CH4) and ethane (C2H6).
- Trigonal pyramidal: A trigonal pyramidal molecule has a central atom with three surrounding atoms arranged in a pyramid, and one lone pair of electrons. Examples include ammonia (NH3) and phosphine (PH3).
- Bent: A bent molecule has a central atom with two surrounding atoms and one or two lone pairs of electrons that cause the molecule to bend. Examples include water (H2O) and sulfur dioxide (SO2).
- Cyclical: A cyclic molecule has a ring structure formed by carbon atoms, and can be planar or non-planar. Examples include benzene (C6H6) and cyclohexane (C6H12).
These are just a few examples of the many shapes that simple organic molecules can take. The shape of a molecule plays an important role in determining its properties and reactivity.
What is Required Basic Principles of Organic Chemistry Shapes of simple organic molecules
The basic principles of organic chemistry that govern the shapes of simple organic molecules are based on the behavior of electrons in the chemical bonds that hold the atoms together.
- Valence shell electron pair repulsion (VSEPR) theory: This theory states that the shape of a molecule is determined by the repulsion between the electron pairs in its valence shell. The electrons in the valence shell want to be as far apart as possible to minimize repulsion, which leads to specific geometric arrangements of the atoms in the molecule.
- Hybridization: Hybridization is the process by which atomic orbitals combine to form hybrid orbitals that are better suited for forming covalent bonds. The shape of a molecule is determined by the hybrid orbitals that the central atom uses to form bonds with the surrounding atoms.
- Molecular orbital theory: This theory describes how the electrons in a molecule are distributed among its molecular orbitals, which are formed by the overlap of atomic orbitals. The shape of a molecule is influenced by the energy levels of its molecular orbitals and the distribution of electrons within them.
- Isomerism: Isomers are molecules that have the same chemical formula but different arrangements of atoms. The shape of a molecule can have a significant impact on its properties and reactivity, and isomers can exhibit different chemical and physical properties due to their different shapes.
These principles are essential for understanding the shapes of simple organic molecules and predicting their properties and behavior in chemical reactions. By applying these principles, chemists can design and synthesize new molecules with specific shapes and properties for a variety of applications.
When is Required Basic Principles of Organic Chemistry Shapes of simple organic molecules
The basic principles of organic chemistry that govern the shapes of simple organic molecules are required in a variety of contexts, including:
- Predicting the properties and reactivity of organic compounds: The shape of a molecule can have a significant impact on its properties, such as boiling point, melting point, solubility, and reactivity. By understanding the basic principles of organic chemistry, chemists can predict the properties and behavior of organic compounds based on their shapes.
- Designing and synthesizing new organic compounds: Chemists often design and synthesize new organic compounds for a variety of applications, such as drugs, materials, and catalysts. By applying the basic principles of organic chemistry, chemists can design molecules with specific shapes and properties to suit their intended applications.
- Understanding the mechanisms of organic reactions: Organic reactions involve the breaking and forming of chemical bonds, which can be influenced by the shapes of the molecules involved. By understanding the basic principles of organic chemistry, chemists can predict and explain the mechanisms of organic reactions based on the shapes of the molecules involved.
- Identifying and characterizing organic compounds: The shape of a molecule can provide important clues about its identity and structure. By using various analytical techniques, such as spectroscopy and chromatography, chemists can identify and characterize organic compounds based on their shapes and other properties.
In summary, the basic principles of organic chemistry that govern the shapes of simple organic molecules are required in a wide range of contexts, from predicting the properties of organic compounds to designing new molecules for various applications.
Where is Required Basic Principles of Organic Chemistry Shapes of simple organic molecules
The basic principles of organic chemistry that govern the shapes of simple organic molecules are applied in various fields, including:
- Organic chemistry research labs: Organic chemists use these principles to design and synthesize new organic compounds for various applications, to study the properties and reactivity of organic molecules, and to develop new methods for organic synthesis.
- Pharmaceutical industry: The basic principles of organic chemistry are essential in the development of new drugs, where the shape of a molecule can affect its ability to interact with biological targets, such as enzymes and receptors.
- Materials science: The basic principles of organic chemistry are applied in the design and synthesis of new materials with specific properties, such as polymers, plastics, and composites.
- Chemical manufacturing: The basic principles of organic chemistry are applied in the production of various chemicals, such as fuels, solvents, and plastics, where the shape of a molecule can affect its properties and behavior during chemical reactions.
- Analytical chemistry labs: The basic principles of organic chemistry are applied in the identification and characterization of organic compounds using various analytical techniques, such as spectroscopy and chromatography.
Overall, the basic principles of organic chemistry that govern the shapes of simple organic molecules are used in a wide range of industries and fields, where organic compounds play a significant role in various applications.
How is Required Basic Principles of Organic Chemistry Shapes of simple organic molecules
The basic principles of organic chemistry that govern the shapes of simple organic molecules can be applied in several ways, including:
- Predicting the geometry of molecules: By using the Valence Shell Electron Pair Repulsion (VSEPR) theory, chemists can predict the geometry of molecules based on the number of electron pairs around the central atom. For example, a molecule with four electron pairs around the central atom has a tetrahedral geometry.
- Determining the hybridization of atoms: Hybridization is used to explain the geometry of molecules and the types of bonds they form. By applying hybridization theory, chemists can determine the hybridization state of atoms in a molecule, which helps predict the geometry of the molecule and its properties.
- Understanding the electronic structure of molecules: By using molecular orbital theory, chemists can understand how electrons are distributed in a molecule and how they contribute to the properties and reactivity of the molecule.
- Identifying and characterizing isomers: Isomers are molecules that have the same chemical formula but different structures and properties. By understanding the basic principles of organic chemistry, chemists can identify and characterize different types of isomers, such as geometric isomers and stereoisomers, based on their different shapes.
- Designing new molecules with specific properties: By using the principles of organic chemistry, chemists can design and synthesize new molecules with specific shapes and properties for various applications, such as drugs, materials, and catalysts.
In summary, the basic principles of organic chemistry that govern the shapes of simple organic molecules are applied in various ways, from predicting the geometry of molecules to designing new molecules with specific properties. By applying these principles, chemists can understand the behavior of organic compounds and develop new compounds for various applications.
Production of Basic Principles of Organic Chemistry Shapes of simple organic molecules
The basic principles of organic chemistry that govern the shapes of simple organic molecules are based on fundamental concepts in chemistry, including the structure of atoms, bonding, and molecular geometry. These principles have been developed and refined over many years through experimental observations and theoretical models.
In terms of the production of these principles, they are typically taught in chemistry courses at the high school and college level, where students learn about the fundamental concepts of organic chemistry, including the properties and reactivity of organic compounds, as well as the different types of chemical bonds, hybridization, and molecular geometry.
Researchers in the field of organic chemistry also contribute to the development and refinement of these principles through their experimental studies and theoretical models. They use various tools and techniques, such as spectroscopy, chromatography, and computational chemistry, to study the properties and behavior of organic compounds and develop new theories and models to explain their observations.
Overall, the production of the basic principles of organic chemistry that govern the shapes of simple organic molecules involves a combination of experimental observations, theoretical models, and education and training in chemistry. Through ongoing research and education, these principles continue to be refined and expanded to better understand the behavior of organic compounds and develop new compounds for various applications.
Case Study on Basic Principles of Organic Chemistry Shapes of simple organic molecules
Case Study: The Importance of Understanding the Shapes of Organic Molecules in Drug Design
One practical application of the basic principles of organic chemistry that govern the shapes of simple organic molecules is in drug design. The shape of a molecule can play a critical role in determining its ability to interact with specific biological targets, such as enzymes and receptors. In this case study, we will examine the role of molecular shape in drug design using the example of the anti-cancer drug, Taxol.
Taxol is a natural product originally isolated from the bark of the Pacific yew tree. It is a complex organic molecule with a unique structure that includes several fused rings and a long, flexible side chain. Taxol was discovered to have potent anti-cancer activity, particularly against ovarian and breast cancers, and has since become an important drug in cancer treatment.
The mechanism of action of Taxol is based on its ability to inhibit the division of cancer cells by binding to a protein called tubulin, which is involved in the formation of the microtubules that help to separate the chromosomes during cell division. Taxol binds to tubulin and stabilizes the microtubules, preventing their disassembly and disrupting the cell division process.
The key to the activity of Taxol lies in its molecular shape. The long, flexible side chain of Taxol allows it to bind to tubulin in a specific orientation, forming a stable complex that inhibits tubulin’s function. The shape of the molecule also plays a role in its solubility and bioavailability, which are critical factors in drug design.
The success of Taxol as an anti-cancer drug highlights the importance of understanding the shapes of organic molecules in drug design. By designing molecules with specific shapes and functional groups, chemists can target specific biological targets and optimize the activity and properties of the drug. The basic principles of organic chemistry, including the VSEPR theory, hybridization, and molecular orbital theory, are essential in predicting and understanding the shapes of organic molecules and their interactions with biological targets.
In conclusion, the case of Taxol demonstrates the importance of understanding the shapes of organic molecules in drug design and the critical role that basic principles of organic chemistry play in this process. By applying these principles, chemists can design and synthesize new drugs with improved activity and properties for the treatment of a variety of diseases, including cancer.
White paper on Basic Principles of Organic Chemistry Shapes of simple organic molecules
White Paper: Understanding the Basic Principles of Organic Chemistry Shapes of Simple Organic Molecules
Introduction
Organic chemistry is the study of carbon-based compounds and their properties, structures, and reactions. One of the key aspects of organic chemistry is the study of the shapes of simple organic molecules, which are critical in understanding the properties and behavior of these compounds. In this white paper, we will explore the basic principles of organic chemistry that govern the shapes of simple organic molecules, including the role of bonding, hybridization, and molecular geometry.
Bonding in Organic Molecules
The structure and properties of organic molecules are determined by the types of bonds they contain. Organic molecules can contain three types of bonds: covalent, polar covalent, and nonpolar covalent. Covalent bonds are formed when two atoms share electrons, while polar covalent bonds are formed when there is an unequal sharing of electrons. Nonpolar covalent bonds are formed when there is an equal sharing of electrons between two atoms.
The type of bonding in an organic molecule can affect its shape and properties. For example, polar covalent bonds can lead to the formation of polar molecules, which have a separation of charge and can exhibit unique properties, such as solubility in water. Nonpolar molecules, on the other hand, are generally insoluble in water and exhibit different properties.
Hybridization of Atomic Orbitals
Another important factor in determining the shape of organic molecules is the hybridization of atomic orbitals. Hybridization is the process by which atomic orbitals mix to form new hybrid orbitals that have different shapes and energies. The most common types of hybridization in organic chemistry are sp, sp2, and sp3.
In sp hybridization, one s and one p orbital combine to form two sp hybrid orbitals, which are linear in shape. In sp2 hybridization, one s and two p orbitals combine to form three sp2 hybrid orbitals, which are trigonal planar in shape. In sp3 hybridization, one s and three p orbitals combine to form four sp3 hybrid orbitals, which are tetrahedral in shape.
Molecular Geometry and VSEPR
Theory The final factor that influences the shape of organic molecules is molecular geometry. Molecular geometry is determined by the arrangement of atoms and electron pairs around the central atom in a molecule. The VSEPR (valence shell electron pair repulsion) theory is used to predict the molecular geometry of simple organic molecules.
According to the VSEPR theory, electron pairs in a molecule repel each other and try to get as far away from each other as possible, leading to specific molecular geometries. For example, a molecule with four electron pairs around the central atom will have a tetrahedral shape, while a molecule with three electron pairs will have a trigonal planar shape.
Conclusion
The basic principles of organic chemistry that govern the shapes of simple organic molecules are critical in understanding the properties and behavior of these compounds. Bonding, hybridization, and molecular geometry all play important roles in determining the shape of an organic molecule, which in turn influences its properties and reactivity. By understanding these principles, chemists can design and synthesize new organic molecules with specific shapes and properties for a wide range of applications, from drug design to materials science.