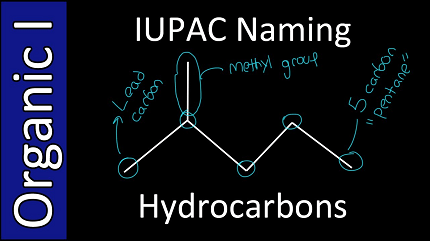
The IUPAC nomenclature of organic molecules is a standardized system used to name organic compounds, including hydrocarbons. The naming system is based on a set of rules that allow chemists to communicate unambiguously about the structure and composition of organic compounds.
The basic principles of the IUPAC nomenclature of hydrocarbons are as follows:
- Identify the longest continuous chain of carbon atoms (the parent chain) in the molecule. This chain may be straight or branched.
- Number the carbon atoms in the parent chain consecutively, starting from the end nearest to any substituents (groups of atoms that are not part of the parent chain).
- Name the substituents as prefixes, indicating their position on the parent chain using the number of the carbon atom to which they are attached. Common substituents include methyl (CH3-), ethyl (C2H5-), propyl (C3H7-), and butyl (C4H9-).
- If there are multiple substituents, list them in alphabetical order, regardless of their position on the chain.
- If there are multiple identical substituents, use prefixes indicating the number of times they occur, such as di- (two), tri- (three), tetra- (four), and so on.
- Indicate the type of hydrocarbon using a suffix based on the number of carbon atoms in the parent chain. For example, an alkane (a hydrocarbon with single bonds only) would have the suffix -ane, while an alkene (a hydrocarbon with one or more carbon-carbon double bonds) would have the suffix -ene.
Examples:
- The hydrocarbon with the formula C6H14 can be named as hexane, since it has a six-carbon parent chain with single bonds only.
- The hydrocarbon with the formula C5H10 can be named as 2-methylbutane, since it has a five-carbon parent chain and a methyl (CH3-) substituent attached to the second carbon atom.
- The hydrocarbon with the formula C4H8 can be named as cyclobutene, since it has a four-carbon ring structure with one carbon-carbon double bond.
What is Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
The basic principles of the IUPAC nomenclature of organic molecules (including hydrocarbons) are as follows:
- Identify the parent chain: The first step in naming an organic molecule is to identify the longest continuous chain of carbon atoms (the parent chain). This chain may be straight or branched.
- Number the carbon atoms: Once the parent chain has been identified, the carbon atoms in the chain must be numbered consecutively, starting from the end nearest to any substituents.
- Identify and name the substituents: Substituents are groups of atoms that are not part of the parent chain. They are identified and named as prefixes, indicating their position on the parent chain using the number of the carbon atom to which they are attached. Common substituents include methyl (CH3-), ethyl (C2H5-), propyl (C3H7-), and butyl (C4H9-).
- Alphabetize the substituents: If there are multiple substituents, they should be listed in alphabetical order, regardless of their position on the chain.
- Use prefixes for multiple substituents: If there are multiple identical substituents, use prefixes indicating the number of times they occur, such as di- (two), tri- (three), tetra- (four), and so on.
- Indicate the type of hydrocarbon: The type of hydrocarbon is indicated by a suffix based on the number of carbon atoms in the parent chain. For example, an alkane (a hydrocarbon with single bonds only) would have the suffix -ane, while an alkene (a hydrocarbon with one or more carbon-carbon double bonds) would have the suffix -ene.
- Combine prefixes and suffixes: The name of the organic molecule is obtained by combining the prefixes and suffixes according to the rules outlined above.
These basic principles are essential for understanding the IUPAC nomenclature of organic molecules and for communicating effectively about the structure and composition of organic compounds.
When is Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
The IUPAC nomenclature of organic molecules (including hydrocarbons) is required whenever chemists need to communicate unambiguously about the structure and composition of organic compounds. This is particularly important in research, industry, and education, where precise and consistent language is essential for understanding and communicating chemical concepts and results.
The IUPAC nomenclature system provides a standardized set of rules for naming organic compounds that can be used by chemists around the world. By following these rules, chemists can ensure that their names accurately reflect the structure and composition of the compounds they are studying or working with, and that they are able to communicate this information clearly and efficiently to others.
In addition, the IUPAC nomenclature system is often used in regulatory contexts, such as in the labeling of chemicals, pharmaceuticals, and food additives. In these cases, accurate and standardized naming is essential for ensuring safety and compliance with regulations.
Where is Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
The Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (including hydrocarbons) are used in various contexts, including research, industry, education, and regulation.
In research, chemists use the IUPAC nomenclature system to accurately and unambiguously name organic compounds they are studying, which is essential for sharing information with colleagues and for publishing research findings.
In industry, the IUPAC nomenclature system is used in the manufacturing and labeling of chemicals, pharmaceuticals, and other products, ensuring that they are accurately named and labeled for safety and regulatory compliance.
In education, the IUPAC nomenclature system is an important part of teaching and learning organic chemistry. Students learn to apply the rules of the nomenclature system to name and identify organic compounds, which is essential for understanding the structure and behavior of these important molecules.
In regulatory contexts, the IUPAC nomenclature system is used to ensure that chemicals, pharmaceuticals, and other products are accurately named and labeled for safety and compliance with regulations. This helps to protect public health and the environment by ensuring that products are used and disposed of safely and appropriately.
How is Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
The Required Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (including hydrocarbons) are applied using a set of standardized rules that ensure consistency and accuracy in naming organic compounds. Here is a brief overview of how these principles are applied:
- Identify the parent chain: The longest continuous chain of carbon atoms in the molecule is identified as the parent chain. This chain may be straight or branched.
- Number the carbon atoms: The carbon atoms in the parent chain are numbered consecutively, starting from the end nearest to any substituents.
- Identify and name the substituents: Substituents are groups of atoms that are not part of the parent chain. They are identified and named as prefixes, indicating their position on the parent chain using the number of the carbon atom to which they are attached.
- Alphabetize the substituents: If there are multiple substituents, they should be listed in alphabetical order, regardless of their position on the chain.
- Use prefixes for multiple substituents: If there are multiple identical substituents, prefixes indicating the number of times they occur, such as di- (two), tri- (three), tetra- (four), and so on are used.
- Indicate the type of hydrocarbon: The type of hydrocarbon is indicated by a suffix based on the number of carbon atoms in the parent chain. For example, an alkane (a hydrocarbon with single bonds only) would have the suffix -ane, while an alkene (a hydrocarbon with one or more carbon-carbon double bonds) would have the suffix -ene.
- Combine prefixes and suffixes: The name of the organic molecule is obtained by combining the prefixes and suffixes according to the rules outlined above.
By following these rules, chemists can accurately name and identify organic compounds, ensuring that they are communicating clearly and consistently with colleagues and other stakeholders in research, industry, education, and regulation.
Nomenclature of Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
The nomenclature system for organic molecules (including hydrocarbons) developed by the International Union of Pure and Applied Chemistry (IUPAC) is based on a set of basic principles. Here is a brief overview of the basic principles of IUPAC nomenclature for organic molecules:
- Longest carbon chain: The longest continuous chain of carbon atoms in the molecule is identified as the parent chain.
- Numbering of carbon chain: The carbon atoms in the parent chain are numbered consecutively, starting from the end nearest to any substituents.
- Identification and naming of substituents: Substituents are groups of atoms that are not part of the parent chain. They are identified and named as prefixes indicating their position on the parent chain using the number of the carbon atom to which they are attached.
- Alphabetical order: If there are multiple substituents, they should be listed in alphabetical order, regardless of their position on the chain.
- Multiple substituents: If there are multiple identical substituents, prefixes indicating the number of times they occur, such as di- (two), tri- (three), tetra- (four), and so on are used.
- Indication of type of hydrocarbon: The type of hydrocarbon is indicated by a suffix based on the number of carbon atoms in the parent chain. For example, an alkane (a hydrocarbon with single bonds only) would have the suffix -ane, while an alkene (a hydrocarbon with one or more carbon-carbon double bonds) would have the suffix -ene.
- Locants: Locants are numbers used to indicate the position of substituents on the parent chain. They are placed immediately before the prefix indicating the substituent.
- Hyphens: Hyphens are used to separate numbers and letters, and to separate prefixes and suffixes.
- Parentheses: Parentheses are used to enclose the locants for substituents when there is ambiguity about which carbon atom they are attached to.
By following these basic principles, chemists can accurately name and identify organic compounds, ensuring that they are communicating clearly and consistently with colleagues and other stakeholders in research, industry, education, and regulation.
Case Study on Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
Case Study: Naming an Organic Molecule using IUPAC Nomenclature
Consider the following organic molecule:
CH3CH2CH(CH3)CH2CH2CH3
Step 1: Identify the parent chain
The parent chain in this molecule is a six-carbon chain, which is the longest continuous chain of carbon atoms. Therefore, the parent chain is a hexane.
Step 2: Number the carbon atoms
The carbon atoms in the parent chain are numbered consecutively, starting from the end nearest to any substituents. In this case, we can number the chain from either end, but we will start from the left-hand side for convenience.
CH3CH2CH(CH3)CH2CH2CH3
1 2 3 4 5 6
Step 3: Identify and name the substituents
The molecule contains a methyl group (CH3) attached to carbon atom 3. Therefore, the substituent is named as a 3-methyl group.
Step 4: Alphabetize the substituents
Since there is only one substituent, there is no need to alphabetize them.
Step 5: Use prefixes for multiple substituents
There is only one substituent in this molecule, so we do not need to use prefixes.
Step 6: Indicate the type of hydrocarbon
The parent chain is a six-carbon chain, so it is a hexane.
Step 7: Combine prefixes and suffixes
Putting it all together, the name of the organic molecule is 3-methylhexane.
Therefore, the IUPAC name of the given organic molecule is 3-methylhexane.
Conclusion:
This case study demonstrates the basic principles of IUPAC nomenclature for organic molecules, which is a standardized system used by chemists to accurately name and identify organic compounds. By following these principles, chemists can communicate clearly and consistently with colleagues and other stakeholders in research, industry, education, and regulation.
White paper on Basic Principles of Organic Chemistry IUPAC nomenclature of organic molecules (hydrocarbons)
Introduction:
Organic chemistry is a branch of chemistry that deals with the study of carbon-containing compounds. Carbon is unique in its ability to form stable covalent bonds with a variety of other elements, including hydrogen, nitrogen, oxygen, and sulfur, among others. The large number of compounds that can be formed from carbon and other elements makes organic chemistry a fascinating and complex field of study. To effectively communicate about these compounds, a standardized system for naming them is necessary. The International Union of Pure and Applied Chemistry (IUPAC) has developed a nomenclature system for organic molecules that is based on a set of basic principles. This white paper will provide an overview of these basic principles and their importance in the field of organic chemistry.
Basic Principles of IUPAC Nomenclature:
The basic principles of IUPAC nomenclature for organic molecules are as follows:
- Longest carbon chain: The longest continuous chain of carbon atoms in the molecule is identified as the parent chain.
- Numbering of carbon chain: The carbon atoms in the parent chain are numbered consecutively, starting from the end nearest to any substituents.
- Identification and naming of substituents: Substituents are groups of atoms that are not part of the parent chain. They are identified and named as prefixes indicating their position on the parent chain using the number of the carbon atom to which they are attached.
- Alphabetical order: If there are multiple substituents, they should be listed in alphabetical order, regardless of their position on the chain.
- Multiple substituents: If there are multiple identical substituents, prefixes indicating the number of times they occur, such as di- (two), tri- (three), tetra- (four), and so on are used.
- Indication of type of hydrocarbon: The type of hydrocarbon is indicated by a suffix based on the number of carbon atoms in the parent chain. For example, an alkane (a hydrocarbon with single bonds only) would have the suffix -ane, while an alkene (a hydrocarbon with one or more carbon-carbon double bonds) would have the suffix -ene.
- Locants: Locants are numbers used to indicate the position of substituents on the parent chain. They are placed immediately before the prefix indicating the substituent.
- Hyphens: Hyphens are used to separate numbers and letters, and to separate prefixes and suffixes.
- Parentheses: Parentheses are used to enclose the locants for substituents when there is ambiguity about which carbon atom they are attached to.
Importance of IUPAC Nomenclature: The importance of IUPAC nomenclature for organic molecules cannot be overstated. The complex structures and vast numbers of organic compounds require a standardized system of naming to ensure that chemists are accurately communicating with each other about the compounds they are working with. Without a standardized system of naming, there would be confusion and miscommunication, which could lead to serious consequences in research, industry, education, and regulation. IUPAC nomenclature provides a consistent and universal language for organic chemistry that allows scientists and other stakeholders to understand each other’s work and build upon it.
Conclusion:
In conclusion, the basic principles of IUPAC nomenclature for organic molecules are a fundamental part of the field of organic chemistry. These principles provide a standardized system of naming that is essential for accurate communication and understanding in research, industry, education, and regulation. By following these principles, chemists can ensure that they are communicating clearly and consistently about the complex and fascinating world of carbon-containing compounds.
