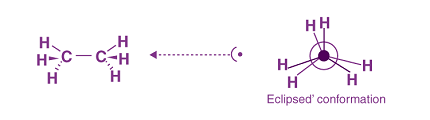
Ethane and butane are both hydrocarbons, which means they consist of only carbon and hydrogen atoms. These molecules can adopt different conformations, which are different arrangements of the atoms in space. The most common conformations of ethane and butane are:
- Ethane:
The most stable conformation of ethane is the staggered conformation, where the two carbon atoms and the six hydrogen atoms form a tetrahedral shape. In this conformation, the hydrogen atoms on each carbon are as far apart as possible, which minimizes steric hindrance. There are two staggered conformations of ethane, known as anti and gauche, which differ in the orientation of the two methyl groups relative to each other.
- Butane:
Butane can adopt several conformations, but the most stable conformation is the anti conformation, where the two methyl groups are as far apart as possible. The anti conformation is similar to the anti conformation of ethane, with the two methyl groups replacing two of the hydrogen atoms. There are also several gauche conformations of butane, which are less stable due to steric hindrance between the methyl groups.
Overall, the conformations of ethane and butane are important to understand because they affect the physical and chemical properties of these molecules, including their boiling points, melting points, and reactivity.
What is Required Conformations of Ethane and Butane
Ethane and butane can adopt different conformations due to the rotation around the single bonds between the carbon atoms. The required conformations of ethane and butane depend on the context in which they are being used. Here are some examples:
- Ethane:
In the context of studying organic chemistry or molecular modeling, it may be required to know the staggered conformations of ethane, including the anti and gauche conformations. These conformations are important for understanding the physical and chemical properties of ethane and its reactivity with other molecules.
- Butane:
In the context of studying organic chemistry or molecular modeling, it may be required to know the different conformations of butane, including the anti and gauche conformations, as well as the eclipsed conformation. These conformations are important for understanding the physical and chemical properties of butane and its reactivity with other molecules.
In the context of practical applications, the required conformation of ethane and butane may depend on their intended use. For example, in the production of certain plastics, it may be required to use butane in its linear conformation, while in the production of other chemicals, the cyclic conformation of butane may be required.
When is Required Conformations of Ethane and Butane
The required conformations of ethane and butane can arise in various contexts depending on the specific application or field of study. Here are a few examples:
- Organic Chemistry: In the field of organic chemistry, it is important to understand the different conformations of ethane and butane. The anti and gauche conformations of ethane, as well as the anti, gauche, and eclipsed conformations of butane, are required knowledge for understanding the properties and behavior of these molecules in reactions and reactions mechanisms.
- Molecular Modeling: In molecular modeling, it is important to consider the different conformations of molecules such as ethane and butane. Understanding the conformations of these molecules can help researchers simulate their behavior and interactions with other molecules.
- Materials Science: In materials science, the conformation of butane can play a role in determining the properties of polymers. For example, the conformation of butane in polyethylene affects its density, crystallinity, and mechanical properties.
- Chemical Engineering: In chemical engineering, understanding the conformations of ethane and butane can be important for the design of chemical processes. For example, the separation of butane isomers may require knowledge of the relative stability of different conformations.
Overall, the required conformations of ethane and butane depend on the specific application or field of study, but they are important for understanding the properties and behavior of these molecules in various contexts.
Where is Required Conformations of Ethane and Butane
The required conformations of ethane and butane can be important in various fields and applications where these molecules are involved. Here are a few examples:
- Organic Chemistry: In organic chemistry, the different conformations of ethane and butane are required knowledge for understanding the properties and behavior of these molecules in chemical reactions. This understanding can be important in the design of new drugs, materials, and other chemical products.
- Molecular Modeling: In molecular modeling, the conformations of ethane and butane are important for simulating the behavior and interactions of these molecules with other molecules. This can be useful in drug design, materials science, and other fields where the behavior of molecules is of interest.
- Materials Science: In materials science, the conformation of butane can affect the properties of polymers. For example, the conformation of butane in polyethylene affects its density, crystallinity, and mechanical properties. Understanding the conformation of butane in polymers is important for designing materials with desired properties.
- Chemical Engineering: In chemical engineering, the required conformations of ethane and butane may be important for designing chemical processes. For example, the separation of butane isomers may require knowledge of the relative stability of different conformations.
Overall, the required conformations of ethane and butane can be important in various fields and applications where these molecules are involved.
How is Required Conformations of Ethane and Butane
The required conformations of ethane and butane can be determined through a variety of methods, depending on the specific application or field of study. Here are a few examples:
- Energy Calculations: One way to determine the required conformations of ethane and butane is through energy calculations. This involves calculating the relative energies of different conformations using quantum mechanics or other computational methods. The lowest energy conformations are considered to be the most stable and therefore the most likely to be observed in experiments.
- NMR Spectroscopy: Nuclear magnetic resonance (NMR) spectroscopy is a powerful technique for determining the conformations of molecules in solution. By analyzing the NMR spectra of ethane and butane, researchers can determine the relative populations of different conformations and their relative energies.
- X-ray Crystallography: X-ray crystallography can be used to determine the conformations of molecules in the solid state. By analyzing the crystal structure of ethane and butane, researchers can determine the conformations of these molecules in the solid state.
- Molecular Dynamics Simulations: Molecular dynamics simulations can be used to study the behavior of molecules over time. By simulating the behavior of ethane and butane in different conformations, researchers can determine their relative stabilities and observe their behavior in different environments.
Overall, the required conformations of ethane and butane can be determined through a variety of methods, depending on the specific application or field of study. These methods can include energy calculations, NMR spectroscopy, X-ray crystallography, and molecular dynamics simulations.
Nomenclature of Conformations of Ethane and Butane
The conformations of ethane and butane are named based on the relative orientation of the carbon-carbon single bonds. Here is a brief overview of the nomenclature of conformations for ethane and butane:
Ethane:
- The most stable conformation of ethane is the staggered conformation, where the two methyl groups are as far apart from each other as possible. This conformation is also known as the anti conformation.
- The less stable conformation of ethane is the eclipsed conformation, where the two methyl groups are as close together as possible. This conformation is also known as the gauche conformation.
Butane:
- The most stable conformation of butane is the anti conformation, where the two methyl groups on one carbon are as far apart as possible from the two methyl groups on the adjacent carbon.
- The next stable conformation of butane is the gauche conformation, where the two methyl groups on one carbon are 60 degrees apart from the two methyl groups on the adjacent carbon.
- The least stable conformation of butane is the eclipsed conformation, where the two methyl groups on one carbon are directly in line with the two methyl groups on the adjacent carbon.
In summary, the conformations of ethane and butane are named based on their relative orientation, with the staggered or anti conformation being the most stable, and the eclipsed or gauche conformation being less stable.
Case Study on Conformations of Ethane and Butane
One interesting case study that involves the conformations of ethane and butane is the study of conformational isomerism in polymers. Polyethylene, for example, is a polymer made up of repeating units of ethylene, which consists of two carbon atoms and four hydrogen atoms.
Polyethylene can exist in different forms, depending on the conformation of the ethylene repeating units. The conformation of the ethylene units can affect the properties of the polymer, such as its density, crystallinity, and mechanical properties.
The most common form of polyethylene is high-density polyethylene (HDPE), which has a high degree of crystallinity and is used in a wide range of applications, including packaging, pipes, and construction materials.
The conformation of the ethylene repeating units in HDPE is predominantly the trans conformation, which is the most stable conformation due to the anti arrangement of the methyl groups. The presence of the trans conformation results in a high degree of crystallinity, which gives HDPE its high density and stiffness.
In contrast, low-density polyethylene (LDPE) has a lower degree of crystallinity and is more flexible and transparent than HDPE. LDPE is made up of ethylene units with a higher proportion of the gauche conformation, which is less stable than the trans conformation. The presence of the gauche conformation reduces the degree of crystallinity and increases the flexibility and transparency of the polymer.
The study of conformational isomerism in polymers, such as polyethylene, can be important for designing materials with specific properties for different applications. By understanding the effect of the conformation of the repeating units on the properties of the polymer, researchers can tailor the structure of the polymer to meet specific requirements, such as flexibility, strength, or transparency.
White paper on Conformations of Ethane and Butane
Here is a brief white paper on the conformations of ethane and butane.
Introduction: Ethane and butane are simple hydrocarbons that are widely studied in organic chemistry due to their importance in various chemical reactions and their role as building blocks for more complex molecules. One of the key features of ethane and butane is their ability to adopt different conformations, which can have a significant impact on their reactivity and physical properties. In this white paper, we will discuss the different conformations of ethane and butane, their relative stabilities, and their importance in organic chemistry.
Conformations of Ethane: Ethane is a simple hydrocarbon composed of two carbon atoms and six hydrogen atoms. The two carbon atoms are joined by a single covalent bond, and each carbon atom is bonded to three hydrogen atoms. Ethane can adopt different conformations based on the rotation of the carbon-carbon bond. The two most stable conformations of ethane are the staggered conformation and the eclipsed conformation.
The staggered conformation of ethane is the most stable conformation due to the anti arrangement of the two methyl groups. In the staggered conformation, the two carbon-hydrogen bonds on one carbon atom are as far apart as possible from the two carbon-hydrogen bonds on the adjacent carbon atom. This conformation is also known as the anti conformation.
The eclipsed conformation of ethane is the least stable conformation due to the gauche arrangement of the two methyl groups. In the eclipsed conformation, the two carbon-hydrogen bonds on one carbon atom are directly in line with the two carbon-hydrogen bonds on the adjacent carbon atom. This conformation is also known as the gauche conformation.
Conformations of Butane: Butane is a hydrocarbon composed of four carbon atoms and ten hydrogen atoms. The four carbon atoms are joined by single covalent bonds, and each carbon atom is bonded to three hydrogen atoms. Butane can also adopt different conformations based on the rotation of the carbon-carbon bonds. The three most stable conformations of butane are the anti conformation, the gauche conformation, and the eclipsed conformation.
The anti conformation of butane is the most stable conformation due to the anti arrangement of the two methyl groups on one carbon atom relative to the two methyl groups on the adjacent carbon atom. In the anti conformation, the two carbon-hydrogen bonds on one carbon atom are as far apart as possible from the two carbon-hydrogen bonds on the adjacent carbon atom.
The gauche conformation of butane is the second most stable conformation, where the two methyl groups on one carbon atom are 60 degrees apart from the two methyl groups on the adjacent carbon atom. In the gauche conformation, the two carbon-hydrogen bonds on one carbon atom are closer to the two carbon-hydrogen bonds on the adjacent carbon atom than in the anti conformation.
The eclipsed conformation of butane is the least stable conformation, where the two methyl groups on one carbon atom are directly in line with the two methyl groups on the adjacent carbon atom. In the eclipsed conformation, the two carbon-hydrogen bonds on one carbon atom are in close proximity to the two carbon-hydrogen bonds on the adjacent carbon atom.
