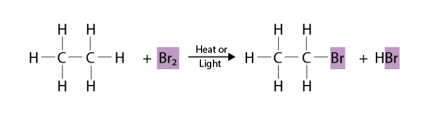
Halogenation refers to a chemical reaction in which a halogen atom (such as chlorine, bromine, or iodine) is introduced into a molecule. This can occur through several different types of reactions, including substitution, addition, and radical reactions.
In a substitution reaction, a halogen atom replaces another atom or group of atoms in a molecule. For example, in the halogenation of methane with chlorine, one of the hydrogen atoms in the methane molecule is replaced by a chlorine atom:
CH4 + Cl2 → CH3Cl + HCl
In an addition reaction, a halogen molecule is added to a carbon-carbon double bond or triple bond, resulting in the formation of a halogenated alkane or alkene, respectively. For example, in the halogenation of ethene with bromine, the double bond is broken and replaced with two single bonds to bromine atoms:
C2H4 + Br2 → C2H4Br2
In a radical reaction, halogen atoms are introduced into a molecule through the use of a halogen radical, which is a highly reactive species that can be generated by the use of heat, light, or a catalyst. For example, in the radical halogenation of methane with chlorine, the chlorine radical is generated and reacts with methane to produce a methyl radical and a hydrogen chloride molecule:
CH4 + Cl• → CH3• + HCl CH3• + Cl2 → CH3Cl + Cl•
What is Required Halogenation
In order for alkanes to undergo halogenation, several conditions must be met:
- Presence of halogens: There must be a source of halogen atoms, such as chlorine or bromine, that can react with the alkane.
- UV light or heat: The halogenation reaction typically requires a source of energy, such as ultraviolet light or heat, to initiate the reaction. This is because the reaction involves breaking a strong carbon-hydrogen bond, which requires a significant amount of energy.
- Presence of an initiator: In some cases, an initiator is needed to generate free radicals that can react with the halogens. Common initiators include peroxides or metals like iron or aluminum.
- Alkane structure: The structure of the alkane can affect its reactivity towards halogens. Alkanes with tertiary carbons or more alkyl groups tend to react more readily than primary or secondary alkanes.
Overall, the conditions required for alkanes halogenation are dependent on the specific reaction being performed and the properties of the alkane being used.
Who is Required Halogenation
Halogenation is a chemical process that can be used in various industries and applications, and there is no specific person or individual who is “required” for halogenation to occur.
Halogenation reactions can be performed by chemists, researchers, and engineers in a laboratory setting to synthesize new compounds or materials. They can also occur naturally in certain environments, such as when sunlight reacts with halogenated hydrocarbons in the atmosphere, leading to the formation of pollutants like smog.
In the industrial sector, halogenation reactions can be used to produce a variety of products, such as plastics, solvents, and pharmaceuticals. For example, the halogenation of benzene is a key step in the production of many important chemicals, including chlorobenzene, which is used as a solvent and in the production of other chemicals.
Overall, halogenation is an important chemical process that has numerous applications across various industries and fields of study, and is carried out by many different individuals and organizations.
When is Required Halogenation
Halogenation can be required or utilized in various situations depending on the specific needs and objectives. Some common instances when halogenation may be required or used include:
- Synthesis of halogenated organic compounds: Halogenation reactions can be used to introduce halogen atoms into organic molecules, producing a range of halogenated compounds that may have useful properties such as improved stability, reactivity, or solubility.
- Production of plastics and other materials: Halogenation is a key step in the production of many types of polymers and plastics, including PVC (polyvinyl chloride), which is widely used in construction and packaging industries.
- Water purification: Halogenation, particularly chlorination, can be used to disinfect water by killing bacteria and other microorganisms. This is commonly used in municipal water treatment facilities to ensure safe drinking water.
- Chemical manufacturing: Halogenation reactions are used in the production of a wide range of chemicals, including pharmaceuticals, pesticides, and solvents.
- Atmospheric chemistry: Halogenation reactions can occur naturally in the atmosphere, particularly in regions with high levels of sunlight and halogenated hydrocarbons. These reactions can lead to the formation of pollutants such as ozone and smog.
Overall, halogenation can be required or utilized in a wide range of situations depending on the specific application and objectives.
Where is Required Halogenation
Halogenation can be required or utilized in various locations and settings, depending on the specific application and objectives. Some common places where halogenation may be required or used include:
- Laboratories: Halogenation reactions are commonly carried out in laboratory settings, where chemists and researchers can control reaction conditions and optimize the process for a specific application.
- Industrial facilities: Halogenation reactions may be used in industrial settings to produce chemicals, plastics, and other materials on a large scale.
- Water treatment facilities: Halogenation, particularly chlorination, is commonly used in municipal water treatment facilities to disinfect water and ensure safe drinking water.
- Agricultural settings: Halogenated compounds such as pesticides and herbicides may be used in agricultural settings to control pests and enhance crop growth.
- Atmospheric environment: Halogenation reactions can occur naturally in the atmosphere, particularly in regions with high levels of sunlight and halogenated hydrocarbons. These reactions can lead to the formation of pollutants such as ozone and smog in the air we breathe.
Overall, halogenation can be required or utilized in a variety of locations and settings, depending on the specific application and objectives.
How is Required Halogenation
Halogenation is a chemical process that involves the addition of a halogen atom to an organic molecule. The exact method for carrying out halogenation can vary depending on the specific application and the type of halogen being used. However, there are some general steps that are typically involved in a halogenation reaction:
- Selection of reagents: The type of halogen and the reaction conditions must be carefully selected based on the desired outcome of the reaction.
- Initiation: Halogenation reactions often require initiation, which can be accomplished through the use of heat or light. The initiation step creates free radicals, which are highly reactive species that can react with the halogen.
- Reaction: The halogen reacts with the organic molecule, forming a new compound that contains the halogen atom. The reaction can be exothermic, meaning that it releases heat, or endothermic, meaning that it absorbs heat.
- Workup: Once the reaction is complete, the resulting product must be isolated and purified. This typically involves separating the product from any unreacted starting material or side products, using techniques such as distillation or chromatography.
Overall, the exact method for carrying out halogenation can vary depending on the specific application and the desired outcome of the reaction. However, the general steps of selection of reagents, initiation, reaction, and workup are typically involved in most halogenation reactions.
Case Study on Halogenation
Here is a case study on the use of halogenation in the synthesis of a pharmaceutical compound:
Case study: Halogenation in the synthesis of a pharmaceutical compound
The pharmaceutical industry is one of the major areas where halogenation is extensively used. One example of halogenation in pharmaceutical synthesis is the synthesis of the drug omeprazole, which is used to treat gastrointestinal disorders such as gastroesophageal reflux disease.
The synthesis of omeprazole involves several steps, including halogenation of a key intermediate compound. The intermediate compound, 5-methoxy-2-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methylsulfinyl]-1H-benzimidazole, is halogenated at the 2-position of the pyridine ring using N-bromosuccinimide (NBS) as the halogenating agent. The reaction is carried out in a chloroform solvent at room temperature, with the addition of a small amount of benzoyl peroxide as a radical initiator.
The halogenation reaction proceeds via a radical mechanism, in which the NBS generates a bromine radical that attacks the 2-position of the pyridine ring. The resulting intermediate radical can then react with molecular oxygen to form a peroxide intermediate, which ultimately leads to the formation of the desired brominated product.
After the halogenation reaction is complete, the product is purified using a combination of solvent extraction and chromatography techniques. The purified product is then further processed to complete the synthesis of omeprazole.
Overall, this case study illustrates how halogenation can be used as a key step in the synthesis of a pharmaceutical compound. The use of halogenation allows for the introduction of specific functional groups into the intermediate compound, which can be critical for achieving the desired properties and efficacy of the final drug product.
White paper on Halogenation
Here is a white paper on halogenation:
Introduction
Halogenation is a chemical process that involves the addition of a halogen atom to an organic molecule. Halogens are a group of elements that includes fluorine, chlorine, bromine, iodine, and astatine. Halogenation is an important tool in synthetic chemistry, as it can be used to introduce specific functional groups into organic molecules, which can be critical for achieving desired properties and reactivity. Halogenation is used in a variety of applications, including the production of pharmaceuticals, pesticides, plastics, and other materials.
Types of Halogenation
There are several different types of halogenation reactions, each of which involves the addition of a halogen to an organic molecule in a different way. Some of the most common types of halogenation reactions include:
- Electrophilic halogenation: This type of halogenation involves the addition of a halogen to an organic molecule in the presence of an electrophilic halogenating agent, such as a halogen gas or a halogen-containing compound. The reaction proceeds via an electrophilic substitution mechanism, in which the halogenating agent attacks the organic molecule and displaces a hydrogen atom.
- Radical halogenation: This type of halogenation involves the addition of a halogen to an organic molecule in the presence of a radical initiator, such as peroxides or light. The reaction proceeds via a radical mechanism, in which the halogenating agent generates a halogen radical that attacks the organic molecule and forms a new radical intermediate.
- Nucleophilic halogenation: This type of halogenation involves the addition of a halogen to an organic molecule in the presence of a nucleophilic halogenating agent, such as an alkali metal halide. The reaction proceeds via a nucleophilic substitution mechanism, in which the halide ion attacks the organic molecule and displaces a leaving group.
Applications of Halogenation
Halogenation is used in a variety of applications, including the production of pharmaceuticals, pesticides, plastics, and other materials. Some of the specific applications of halogenation include:
- Production of pharmaceuticals: Halogenation is a key step in the synthesis of many pharmaceutical compounds, including anti-inflammatory drugs, anti-viral drugs, and anti-cancer drugs. Halogenation can be used to introduce specific functional groups into the intermediate compounds, which can be critical for achieving desired properties and efficacy.
- Production of pesticides: Halogenated compounds are often used as pesticides and herbicides, as they can be effective in controlling pests and enhancing crop growth. However, some halogenated pesticides have been found to be harmful to human health and the environment.
- Production of plastics: Halogenated compounds, such as polyvinyl chloride (PVC), are used in the production of a variety of plastics. However, the production and disposal of halogenated plastics can have negative environmental impacts.
- Water treatment: Halogenation, particularly chlorination, is commonly used in municipal water treatment facilities to disinfect water and ensure safe drinking water.
Conclusion
Halogenation is an important tool in synthetic chemistry, as it allows for the introduction of specific functional groups into organic molecules. Halogenation is used in a variety of applications, including the production of pharmaceuticals, pesticides, plastics, and other materials. However, the production and disposal of halogenated compounds can have negative environmental impacts, and there is ongoing research into the development of more sustainable and environmentally friendly alternatives.
