Boiling point is a physical property of a substance that refers to the temperature at which the substance changes from a liquid state to a gaseous state. It is determined by the strength of intermolecular forces within the substance and the atmospheric pressure.
Some examples of boiling points of common substances are:
- Water: 100°C (at standard atmospheric pressure)
- Ethanol: 78.37°C
- Acetone: 56.05°C
- Benzene: 80.1°C
- Chloroform: 61.15°C
- Carbon dioxide: -78.5°C (at standard atmospheric pressure)
- Nitrogen: -196°C (at standard atmospheric pressure)
- Oxygen: -183°C (at standard atmospheric pressure)
It’s worth noting that the boiling point of a substance can vary depending on the pressure it is under. For example, water will boil at a lower temperature at high altitudes where the atmospheric pressure is lower. Additionally, impurities or changes in the chemical structure of a substance can also affect its boiling point.
What is Required Alkenes and Alkynes Physical properties (boiling points)
The boiling points of alkenes and alkynes are generally lower than those of corresponding alkanes with similar molecular weights. This is due to the fact that alkenes and alkynes have weaker intermolecular forces of attraction than alkanes.
Here are some examples of boiling points of common alkenes and alkynes:
Alkenes:
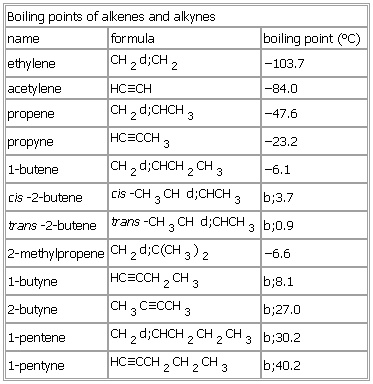
- Ethene: -103.7°C
- Propene: -47.6°C
- Butene: -6.3°C
- Pentene: 30.3°C
- Hexene: 68.7°C
- Heptene: 118.1°C
- Octene: 126.1°C
- Nonene: 151.9°C
- Decene: 174.1°C
Alkynes:
- Ethyne (acetylene): -84°C
- Propyne: -23.2°C
- Butyne: 8.9°C
- Pentyne: 36.1°C
- Hexyne: 69.6°C
- Heptyne: 111°C
- Octyne: 124°C
- Nonyne: 147°C
- Decyne: 178°C
It’s important to note that the boiling points of alkenes and alkynes can vary depending on factors such as molecular weight, branching, and the presence of functional groups.
When is Required Alkenes and Alkynes Physical properties (boiling points)
Alkenes and alkynes are types of hydrocarbons that contain carbon-carbon double and triple bonds, respectively. These bonds have different bond energies and shapes compared to carbon-carbon single bonds found in alkanes, which can affect their physical properties, such as boiling points.
Generally, the boiling points of alkenes and alkynes are lower than those of corresponding alkanes with similar molecular weights. This is due to the fact that alkenes and alkynes have weaker intermolecular forces of attraction than alkanes. The strength of these intermolecular forces depends on factors such as the surface area of the molecule and the polarizability of the electrons within the molecule.
The presence of the double and triple bonds in alkenes and alkynes respectively reduces the surface area of the molecule, which results in weaker intermolecular forces of attraction. This, in turn, lowers the boiling point of the compound. Additionally, the presence of the double and triple bonds can also affect the shape of the molecule, leading to differences in the polarizability of the electrons.
It’s important to note that the boiling points of alkenes and alkynes can vary depending on factors such as molecular weight, branching, and the presence of functional groups. However, in general, alkenes and alkynes tend to have lower boiling points than alkanes with similar molecular weights.
Where is Required Alkenes and Alkynes Physical properties (boiling points)
The boiling points of alkenes and alkynes, like other physical properties, can be found in various reference materials such as textbooks, scientific journals, and online databases.
For example, the CRC Handbook of Chemistry and Physics is a well-known reference book that contains a wealth of information on the physical and chemical properties of compounds, including alkenes and alkynes. Online databases such as PubChem and ChemSpider also provide information on the boiling points of various compounds, including alkenes and alkynes.
In addition to reference materials, experimental techniques such as distillation can be used to measure the boiling points of alkenes and alkynes. Distillation is a technique used to separate a mixture of compounds based on their boiling points. By heating the mixture, the compound with the lowest boiling point will vaporize first, and can be collected and separated from the mixture. This process can be repeated for higher boiling point compounds in the mixture. The boiling points of the collected fractions can be measured experimentally using techniques such as gas chromatography or infrared spectroscopy.
It’s worth noting that the boiling points of alkenes and alkynes can vary depending on factors such as molecular weight, branching, and the presence of functional groups, so it’s important to consult reference materials or perform experiments specific to the compound in question.
How is Required Alkenes and Alkynes Physical properties (boiling points)
The boiling points of alkenes and alkynes are influenced by various factors such as molecular weight, degree of branching, and the presence of functional groups. In general, the boiling points of alkenes and alkynes are lower than those of corresponding alkanes with similar molecular weights.
One reason for this is that alkenes and alkynes have weaker intermolecular forces of attraction than alkanes. This is due to the fact that the π-electrons in the double and triple bonds of alkenes and alkynes are more delocalized than the σ-electrons in alkanes, resulting in a weaker intermolecular attraction.
Additionally, alkenes and alkynes have a lower surface area than alkanes with similar molecular weights, which also contributes to the weaker intermolecular forces of attraction and lower boiling points.
The degree of branching in an alkene or alkyne can also affect its boiling point. Branched alkenes and alkynes have a lower surface area than their straight-chain counterparts, leading to weaker intermolecular forces and lower boiling points.
The presence of functional groups, such as halogens or oxygen-containing groups, can also influence the boiling point of an alkene or alkyne. Functional groups can increase the polarity of the molecule, resulting in stronger intermolecular forces and higher boiling points.
It’s important to note that the boiling points of alkenes and alkynes can vary depending on factors such as molecular weight, degree of branching, and the presence of functional groups. Therefore, to accurately determine the boiling point of a specific alkene or alkyne, it is necessary to consult reference materials or perform experimental measurements.
Production of Alkenes and Alkynes Physical properties (boiling points)
Alkenes and alkynes can be produced by various methods, such as cracking of petroleum or by chemical reactions like dehydrogenation of alkanes, elimination reactions of haloalkanes, and oxidation of alcohols.
The physical properties, such as boiling points, of alkenes and alkynes can vary depending on the specific compound. However, in general, alkenes and alkynes have lower boiling points than their corresponding alkanes with similar molecular weights due to the weaker intermolecular forces of attraction resulting from their double and triple bonds.
The boiling points of alkenes and alkynes can also be affected by factors such as molecular weight, degree of branching, and the presence of functional groups. As molecular weight increases, the boiling point generally increases as well due to the increased intermolecular forces of attraction. However, branching in the carbon chain can decrease the boiling point due to the decreased surface area of the molecule. The presence of functional groups can also increase the boiling point due to the increased polarity of the molecule.
It’s important to note that the boiling points of alkenes and alkynes can vary greatly depending on the specific compound, so it’s necessary to consult reference materials or perform experimental measurements to accurately determine the boiling point of a particular compound.
Case Study on Alkenes and Alkynes Physical properties (boiling points)
A case study on the physical properties of alkenes and alkynes can be seen in the comparison of boiling points between ethene and ethyne.
Ethene (C2H4) is a colorless gas that is used widely in industry, especially in the production of plastics. Ethyne (C2H2), also known as acetylene, is a colorless gas that is used in welding and as a fuel for cutting and welding torches.
The boiling point of ethene is -103.7°C, while the boiling point of ethyne is -84°C. This difference in boiling point can be attributed to the presence of the triple bond in ethyne, which results in weaker intermolecular forces of attraction than in ethene.
The triple bond in ethyne leads to a more compact molecule and fewer points of contact between the molecules. As a result, the intermolecular forces of attraction between the molecules are weaker in ethyne than in ethene, leading to a lower boiling point.
It’s worth noting that other factors can also influence the boiling points of alkenes and alkynes, such as molecular weight, degree of branching, and the presence of functional groups. However, in the case of ethene and ethyne, the main factor influencing the difference in boiling point is the presence of the triple bond in ethyne.
Overall, the physical properties of alkenes and alkynes can vary greatly depending on the specific compound and factors such as molecular weight, degree of branching, and the presence of functional groups. It’s important to consider these factors when studying the physical properties of alkenes and alkynes.
White paper on Alkenes and Alkynes Physical properties (boiling points)
Introduction:
Alkenes and alkynes are important classes of organic compounds with diverse applications in industry and daily life. Understanding the physical properties of these compounds, including their boiling points, is essential for various applications, such as chemical synthesis, separation, and purification. This white paper discusses the physical properties of alkenes and alkynes, with a particular focus on their boiling points.
Boiling points of Alkenes:
Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. Due to the presence of a double bond, the boiling points of alkenes are generally lower than those of corresponding alkanes with similar molecular weights. The lower boiling points of alkenes are due to their weaker intermolecular forces of attraction, which results from the delocalization of π-electrons in the double bond. The degree of branching in an alkene can also affect its boiling point, with branched alkenes having lower boiling points due to their lower surface area.
Boiling points of Alkynes:
Alkynes are unsaturated hydrocarbons that contain at least one carbon-carbon triple bond. The presence of a triple bond in alkynes leads to even weaker intermolecular forces of attraction than those in alkenes, resulting in lower boiling points. The triple bond in alkynes also leads to a more compact molecular structure and fewer points of contact between the molecules, further reducing intermolecular forces of attraction. Like alkenes, the boiling points of alkynes can be affected by factors such as degree of branching and functional groups.
Factors Affecting Boiling Points of Alkenes and Alkynes:
The boiling points of alkenes and alkynes can be influenced by several factors, including molecular weight, degree of branching, and the presence of functional groups. Molecular weight is one of the most significant factors affecting the boiling points of alkenes and alkynes, with heavier compounds having higher boiling points due to increased intermolecular forces of attraction. The degree of branching in a molecule can also affect its boiling point, with branched compounds having lower boiling points due to their lower surface area. The presence of functional groups, such as halogens or oxygen-containing groups, can increase the polarity of the molecule and result in stronger intermolecular forces of attraction, leading to higher boiling points.
Applications of Boiling Point of Alkenes and Alkynes:
The boiling points of alkenes and alkynes are important in various applications, such as chemical synthesis and separation. For example, distillation is a common method used to separate mixtures of compounds with different boiling points. By knowing the boiling points of alkenes and alkynes, chemists can design and optimize distillation processes to separate mixtures of these compounds. The boiling points of alkenes and alkynes are also important in the design of various industrial processes, such as the production of polymers and other organic compounds.
Conclusion:
The physical properties of alkenes and alkynes, including their boiling points, are essential to understand for various applications in industry and daily life. The boiling points of alkenes and alkynes are affected by factors such as molecular weight, degree of branching, and the presence of functional groups. By understanding these factors, chemists can design and optimize various processes that involve these compounds, such as distillation and chemical synthesis. Further research in this area can lead to the development of new applications and technologies involving alkenes and alkynes.
