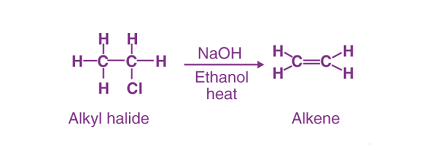
Elimination reactions are a type of organic reaction where a molecule loses one or more atoms or groups of atoms to form a double bond or a triple bond. The most common type of elimination reaction is called a beta-elimination, where the atom or group of atoms that is eliminated is adjacent to a leaving group.
Here are some steps you can follow to prepare for an elimination reaction:
- Understand the reaction mechanism: Elimination reactions can follow different mechanisms, such as E1, E2, or E1cb. It is important to understand the differences between these mechanisms and the factors that influence them, such as the nature of the leaving group, the strength of the base, and the steric hindrance of the substrate.
- Choose the appropriate reagents: Depending on the type of elimination reaction you want to perform, you may need to choose different reagents. For example, E2 reactions require a strong base such as sodium hydroxide or potassium tert-butoxide, while E1 reactions can be catalyzed by acids such as sulfuric acid or phosphoric acid.
- Optimize the reaction conditions: The reaction conditions, such as temperature, solvent, and concentration, can affect the rate and selectivity of the elimination reaction. It is important to optimize these conditions to achieve the desired outcome.
- Purify the product: After the reaction, the product may need to be purified to remove impurities and by-products. This can be done using techniques such as chromatography, distillation, or recrystallization.
- Analyze the product: Finally, the product should be analyzed using spectroscopic techniques such as NMR, IR, or mass spectrometry to confirm its structure and purity.
By following these steps, you can prepare for an elimination reaction and increase your chances of obtaining the desired product.
What is Required Preparation by elimination reactions
The preparation required for an elimination reaction depends on the specific reaction and the desired outcome. However, some general requirements include:
- Suitable substrate: The substrate should have a leaving group that can be eliminated to form a double bond or triple bond. The substrate should also be stable under the reaction conditions.
- Appropriate reagents: The reagents used for the elimination reaction should be chosen based on the type of reaction being performed. For example, E2 reactions require a strong base such as sodium hydroxide or potassium tert-butoxide, while E1 reactions can be catalyzed by acids such as sulfuric acid or phosphoric acid.
- Reaction conditions: The reaction conditions should be optimized to ensure the desired outcome. Factors such as temperature, solvent, and concentration can affect the rate and selectivity of the reaction.
- Purification: After the reaction, the product may need to be purified to remove impurities and by-products. This can be done using techniques such as chromatography, distillation, or recrystallization.
- Analysis: The product should be analyzed using spectroscopic techniques such as NMR, IR, or mass spectrometry to confirm its structure and purity.
In addition to these requirements, it is important to have a good understanding of the reaction mechanism and the factors that influence it. This will help in choosing the appropriate reagents and reaction conditions, and in predicting the outcome of the reaction.
When is Required Preparation by elimination reactions
Elimination reactions can be useful in a variety of situations, such as:
- Synthesis of alkenes and alkynes: Elimination reactions can be used to synthesize alkenes and alkynes from alkyl halides or alcohols. This can be useful in the synthesis of complex organic molecules.
- Formation of cyclic compounds: Elimination reactions can be used to form cyclic compounds by intramolecular elimination. This can be useful in the synthesis of natural products and pharmaceuticals.
- Dehydration of alcohols: Elimination reactions can be used to dehydrate alcohols to form alkenes. This can be useful in the synthesis of polymers and other materials.
- Elimination of leaving groups: Elimination reactions can be used to remove leaving groups from substrates, making them more reactive towards other reactions. This can be useful in the synthesis of complex organic molecules.
In each of these situations, preparation by elimination reactions is required to optimize the reaction conditions, choose the appropriate reagents, and ensure the desired outcome. By understanding the reaction mechanism and the factors that influence it, chemists can prepare for elimination reactions and use them effectively in organic synthesis.
Where is Required Preparation by elimination reactions
Elimination reactions can be performed in a variety of settings, including academic and industrial research laboratories, as well as in production facilities for the synthesis of commercial products.
In academic research laboratories, elimination reactions may be used for the synthesis of new organic compounds and the study of reaction mechanisms. Researchers may also use elimination reactions to modify the properties of existing compounds, such as making them more reactive or altering their physical properties.
In industrial settings, elimination reactions may be used for the production of commercial products such as pharmaceuticals, agrochemicals, and polymers. Industrial chemists must optimize the reaction conditions and choose the appropriate reagents to ensure high yields, low costs, and good purity of the final product.
Elimination reactions can also be used in a variety of applications outside of the laboratory, such as in the manufacture of plastics, synthetic fibers, and other materials. In these settings, elimination reactions must be carefully controlled to ensure consistent quality and optimal efficiency.
Overall, elimination reactions are a widely used tool in organic chemistry and can be found in many different settings, from academic research to industrial production. The required preparation for elimination reactions will vary depending on the specific application and desired outcome.
How is Required Preparation by elimination reactions
The preparation required for an elimination reaction depends on the specific reaction and the desired outcome. However, some general steps for preparation by elimination reactions include:
- Selection of substrate: The substrate should have a leaving group that can be eliminated to form a double bond or triple bond. The substrate should also be stable under the reaction conditions.
- Selection of appropriate reagents: The reagents used for the elimination reaction should be chosen based on the type of reaction being performed. For example, E2 reactions require a strong base such as sodium hydroxide or potassium tert-butoxide, while E1 reactions can be catalyzed by acids such as sulfuric acid or phosphoric acid.
- Optimization of reaction conditions: The reaction conditions should be optimized to ensure the desired outcome. Factors such as temperature, solvent, and concentration can affect the rate and selectivity of the reaction. For example, high temperatures may favor elimination reactions over substitution reactions, but may also lead to side reactions or decomposition of the substrate.
- Purification: After the reaction, the product may need to be purified to remove impurities and by-products. This can be done using techniques such as chromatography, distillation, or recrystallization.
- Analysis: The product should be analyzed using spectroscopic techniques such as NMR, IR, or mass spectrometry to confirm its structure and purity.
In addition to these steps, it is important to have a good understanding of the reaction mechanism and the factors that influence it. This will help in choosing the appropriate reagents and reaction conditions, and in predicting the outcome of the reaction. Computational methods, such as molecular modeling and density functional theory calculations, can also be useful for predicting the reaction outcome and designing new substrates.
Overall, preparation by elimination reactions requires careful selection of substrates and reagents, optimization of reaction conditions, purification, and analysis to ensure the desired outcome. By following these steps, chemists can prepare for elimination reactions and use them effectively in organic synthesis.
Nomenclature of Preparation by elimination reactions
The nomenclature for preparation by elimination reactions depends on the type of elimination reaction being performed. Here are some examples:
- Dehydration of alcohols: When an alcohol undergoes elimination to form an alkene, the reaction is called dehydration. The product is named by replacing the “-ol” ending of the alcohol with “-ene”. For example, the dehydration of ethanol produces ethene.
- Synthesis of alkenes and alkynes: When an alkyl halide or alcohol undergoes elimination to form an alkene or alkyne, the product is named by adding the prefix “-ene” or “-yne” to the name of the parent hydrocarbon. For example, the reaction of 2-chloropropane with a strong base to form propene is named as dehydrohalogenation of 2-chloropropane.
- Cyclic compounds: When a cyclic compound is formed by intramolecular elimination, the product is named by indicating the size of the ring and the location of the double bond or triple bond. For example, the intramolecular elimination of 1,5-dibromopentane produces cyclopentene.
- Elimination of leaving groups: When a leaving group is eliminated from a substrate to form a more reactive species, the product is named based on the structure of the substrate and the type of elimination reaction. For example, the elimination of a leaving group from a benzyl chloride may produce a benzyl cation or a styrene molecule, depending on the reaction conditions.
Overall, the nomenclature for preparation by elimination reactions follows the standard rules for naming organic compounds, with specific modifications based on the type of elimination reaction and the structure of the substrate and product.
Case Study on Preparation by elimination reactions
One example of a case study involving preparation by elimination reactions is the synthesis of butadiene from 1,3-butadiene diol. Butadiene is an important chemical used in the production of synthetic rubbers, such as styrene-butadiene rubber (SBR), which is widely used in the tire industry.
The synthesis of butadiene from 1,3-butadiene diol involves a two-step process. In the first step, the diol undergoes dehydration to form 1,3-butadiene. In the second step, the butadiene is purified and concentrated.
Step 1: Dehydration of 1,3-butadiene diol
The dehydration of 1,3-butadiene diol can be achieved using a strong acid catalyst, such as sulfuric acid. The reaction takes place via an E1 mechanism, in which the leaving group (a water molecule) is eliminated to form a carbocation intermediate, which then undergoes deprotonation to form the alkene.
The reaction can be represented as follows:
1,3-butadiene diol → 1,3-butadiene + H2O
Step 2: Purification and concentration of butadiene
The butadiene product from the first step is a gas and must be purified and concentrated before use. This can be achieved by several methods, including fractional distillation and adsorption.
In fractional distillation, the butadiene is separated from other components in the reaction mixture based on its boiling point. The process involves heating the reaction mixture to vaporize the butadiene, then cooling and condensing it to collect the pure product.
In adsorption, the butadiene is adsorbed onto a solid material, such as activated carbon or molecular sieves, which selectively adsorb the butadiene and allow other components to pass through. The butadiene is then desorbed from the solid material using a solvent, such as acetone or ethanol.
Overall, the synthesis of butadiene from 1,3-butadiene diol involves preparation by elimination reactions, specifically the dehydration of the diol to form the alkene. The process highlights the importance of careful selection of reagents and reaction conditions, as well as the need for effective purification and concentration methods to obtain a high-quality product.
White paper on Preparation by elimination reactions
Introduction:
Preparation by elimination reactions is a fundamental concept in organic chemistry. Elimination reactions involve the removal of a leaving group from a substrate to form a more reactive species, such as an alkene, alkyne, or carbocation. These reactions play a critical role in the synthesis of a wide range of organic compounds, including polymers, pharmaceuticals, and agrochemicals.
Types of elimination reactions:
There are several types of elimination reactions, including:
- Dehydration: In this reaction, an alcohol loses a water molecule to form an alkene.
- Dehydrohalogenation: In this reaction, an alkyl halide loses a halide ion to form an alkene.
- Dehalogenation: In this reaction, a halogenated hydrocarbon loses a halide ion to form an alkene.
- Elimination of leaving groups: In this reaction, a leaving group is eliminated from a substrate to form a more reactive species.
Mechanism of elimination reactions:
Elimination reactions occur via either an E1 or E2 mechanism, depending on the reaction conditions and the structure of the substrate. In an E1 mechanism, the reaction occurs in two steps: first, the leaving group is eliminated to form a carbocation intermediate, and second, a proton is removed from an adjacent carbon to form the double bond. In an E2 mechanism, the reaction occurs in a single step, with the leaving group and a proton being eliminated simultaneously.
Applications of elimination reactions:
Elimination reactions are used extensively in the synthesis of organic compounds. For example, the synthesis of alkenes and alkynes from alkyl halides or alcohols is achieved via elimination reactions. Elimination reactions are also used in the synthesis of polymers, such as polypropylene and polyethylene, which are widely used in the production of plastics. Furthermore, elimination reactions are used in the synthesis of pharmaceuticals and agrochemicals.
Conclusion:
Preparation by elimination reactions is a fundamental concept in organic chemistry, and elimination reactions play a critical role in the synthesis of a wide range of organic compounds. Understanding the mechanism of elimination reactions and their applications is essential for the development of new synthetic methods and the production of high-quality organic compounds.
