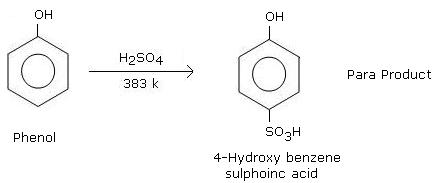
Phenols can be sulphonated by treating them with a mixture of concentrated sulphuric acid and fuming sulphuric acid (oleum). The process involves the substitution of a hydrogen atom on the phenolic ring with a sulphonate (SO3H) group. The sulphonation of phenols is an important reaction in organic chemistry and has several industrial applications, including the production of dyes, pharmaceuticals, and detergents.
The mechanism of sulphonation involves the generation of the electrophilic species, the sulphur trioxide (SO3) which is generated from the oleum. The first step involves the protonation of the phenolic hydroxyl group by the concentrated sulphuric acid to generate a highly reactive electrophile species. The protonated phenol undergoes electrophilic aromatic substitution with the sulphonating agent (SO3) to form the sulphonated phenol.
The sulphonation reaction is highly exothermic and needs to be carefully controlled to prevent overheating and subsequent decomposition of the reactants. The reaction mixture is usually kept at low temperatures and carefully stirred during the addition of the oleum. After completion of the reaction, the sulphonated product is isolated by neutralizing the reaction mixture with a suitable base, such as sodium hydroxide.
Overall, sulphonation is an important reaction for the modification of phenolic compounds, and the process can be used to tailor the physical and chemical properties of these compounds for specific applications.
What is Required Phenols Sulphonation
To carry out the sulphonation of phenols, the following reagents and conditions are required:
- Concentrated sulphuric acid: It is used to protonate the phenolic hydroxyl group and to generate the electrophilic species.
- Fuming sulphuric acid (oleum): It is used as a source of sulphur trioxide (SO3), which is the sulphonating agent.
- Phenol: It is the starting material for the sulphonation reaction. It should be of high purity and should be dried before use.
- Suitable solvent: A suitable solvent, such as dichloromethane or chloroform, may be used to dissolve the phenol and to help maintain the temperature of the reaction.
- Cooling system: The reaction is highly exothermic, and the temperature needs to be carefully controlled to prevent overheating. A cooling system, such as a water bath or ice bath, may be used to maintain the temperature of the reaction.
- Base: After the completion of the reaction, the sulphonated product is isolated by neutralizing the reaction mixture with a suitable base, such as sodium hydroxide.
It is important to note that the sulphonation reaction is highly exothermic and needs to be carefully controlled to prevent overheating and decomposition of the reactants. Special care should be taken while handling the reagents, and appropriate safety measures should be taken to ensure safe handling of the chemicals.
When is Required Phenols Sulphonation
Phenols sulphonation is used in various applications, including the production of dyes, pharmaceuticals, and detergents. The sulphonation reaction is used to introduce a sulphonate group (-SO3H) into the phenol molecule, which modifies its properties and makes it suitable for various applications. Some specific examples of when phenols sulphonation is required are:
- Dye production: Sulphonated phenols are used as intermediates in the production of azo dyes, anthraquinone dyes, and other types of dyes.
- Pharmaceutical industry: Sulphonated phenols are used as intermediates in the synthesis of various pharmaceuticals, including antibiotics, antiseptics, and analgesics.
- Surfactant production: Sulphonated phenols are used in the production of surfactants, which are used in detergents, emulsifiers, and wetting agents.
- Chemical industry: Sulphonated phenols are used in various chemical reactions, including polymerization reactions, oxidation reactions, and reduction reactions.
Overall, phenols sulphonation is an important reaction in organic chemistry and has various industrial applications. The sulphonation reaction allows for the modification of phenolic compounds to tailor their physical and chemical properties for specific applications.
Where is Required Phenols Sulphonation
Phenols sulphonation is a widely used reaction in the chemical industry and has various applications. Some specific examples of where phenols sulphonation is required are:
- Dye industry: Sulphonated phenols are used as intermediates in the production of dyes. The sulphonation of phenols is an essential step in the synthesis of azo dyes, anthraquinone dyes, and other types of dyes.
- Pharmaceutical industry: Sulphonated phenols are used as intermediates in the synthesis of various pharmaceuticals, including antibiotics, antiseptics, and analgesics.
- Surfactant production: Sulphonated phenols are used in the production of surfactants, which are used in detergents, emulsifiers, and wetting agents.
- Chemical industry: Sulphonated phenols are used in various chemical reactions, including polymerization reactions, oxidation reactions, and reduction reactions.
- Petrochemical industry: Sulphonated phenols are used as additives in lubricating oils and as emulsifiers in petroleum refining.
- Textile industry: Sulphonated phenols are used in the dyeing of textiles and as stabilizers for dye baths.
Overall, phenols sulphonation is an important reaction in the chemical industry, and the sulphonated products find various applications in different sectors.
How is Required Phenols Sulphonation
The sulphonation of phenols can be carried out by treating phenol with concentrated sulphuric acid (H2SO4) and fuming sulphuric acid (oleum, H2S2O7). The reaction involves the substitution of a hydrogen atom on the phenolic hydroxyl group with a sulphonate group (-SO3H) to form a sulfonic acid. The general reaction can be represented as:
Phenol + H2SO4 → PhOSO2OH + H2O
The reaction proceeds through an electrophilic aromatic substitution mechanism, where the electrophilic species is the sulphonium ion (HSO4+). The sulphur trioxide (SO3) generated from the fuming sulphuric acid reacts with the phenol to form the sulphonium ion, which then reacts with the phenol to form the sulphonated product.
The reaction is highly exothermic and needs to be carefully controlled to prevent overheating and decomposition of the reactants. The temperature is maintained by using a cooling system, such as a water bath or ice bath. After the completion of the reaction, the sulphonated product is isolated by neutralizing the reaction mixture with a suitable base, such as sodium hydroxide (NaOH).
Overall, the sulphonation of phenols is an important reaction in organic chemistry and has various industrial applications. The sulphonated products have unique physical and chemical properties, which make them useful in various applications.
Nomenclature of Phenols Sulphonation
The nomenclature of sulphonated phenols depends on the position of the sulphonate group (-SO3H) on the phenolic ring. The sulphonation reaction can occur at any position on the phenolic ring, leading to the formation of various isomeric sulphonated products. The position of the sulphonate group is indicated by the prefix ortho-, meta-, or para-, depending on whether it is attached to the 1, 2-, 1, 3-, or 1, 4-positions of the phenolic ring, respectively.
For example, when phenol is sulphonated at the ortho-position, the product is called ortho-sulphonic acid, and the structure is represented as Ph-OSO3H. Similarly, when the sulphonation occurs at the meta-position, the product is called meta-sulphonic acid, and the structure is represented as Ph-SO3H. When the sulphonation occurs at the para-position, the product is called para-sulphonic acid, and the structure is represented as Ph-POSO3H.
In cases where more than one sulphonate group is present on the phenolic ring, the position of each sulphonate group is indicated by a numerical prefix, followed by the position number of the sulphonate group. For example, when phenol is sulphonated at both the ortho-positions, the product is called 2,2′-disulphonic acid, and the structure is represented as Ph-(OSO2OH)2.
Overall, the nomenclature of sulphonated phenols follows the same rules as that of aromatic compounds, with the addition of prefixes indicating the position of the sulphonate group on the phenolic ring.
Case Study on Phenols Sulphonation
Case study: Sulphonation of phenol for the production of phenol-2-sulphonic acid
Phenol-2-sulphonic acid is an important intermediate in the production of various dyes and pigments. In this case study, we will look at the sulphonation of phenol for the production of phenol-2-sulphonic acid.
Process description:
The sulphonation of phenol is carried out by adding concentrated sulphuric acid (H2SO4) to a mixture of phenol and water in a reaction vessel. The reaction mixture is then heated to a temperature of 80-100 °C with constant stirring. Once the temperature stabilizes, fuming sulphuric acid (oleum, H2S2O7) is added dropwise to the reaction mixture. The reaction is highly exothermic, and the temperature is maintained by a cooling system. After the addition of fuming sulphuric acid is complete, the reaction mixture is allowed to cool to room temperature. The resulting mixture is then neutralized with a sodium hydroxide (NaOH) solution to form the phenol-2-sulphonic acid product.
Process conditions:
- Phenol concentration: 10-20% (w/v)
- H2SO4 concentration: 80-90% (v/v)
- Temperature: 80-100 °C
- Reaction time: 2-3 hours
- Cooling system: Water bath
- NaOH concentration: Sufficient to neutralize the acid
Product quality:
The quality of the phenol-2-sulphonic acid product is determined by its purity, yield, and physical properties. The product is typically analyzed by techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and infrared spectroscopy (IR). The purity of the product is typically greater than 90%, and the yield is around 70-80%. The physical properties of the product, such as melting point and solubility, are also important parameters that are monitored.
Application:
Phenol-2-sulphonic acid is used as an intermediate in the production of various dyes and pigments, including acid dyes, reactive dyes, and direct dyes. The sulphonation of phenol to form phenol-2-sulphonic acid is a key step in the synthesis of these dyes. The product is also used as an intermediate in the production of various pharmaceuticals and agrochemicals.
Environmental and safety considerations:
The sulphonation of phenol is a hazardous process that requires strict safety precautions to prevent accidents and environmental pollution. The concentrated sulphuric acid and fuming sulphuric acid used in the reaction are highly corrosive and can cause severe burns if they come into contact with the skin or eyes. The reaction is also highly exothermic, and the temperature needs to be carefully controlled to prevent overheating and decomposition of the reactants. The reaction vessel and the cooling system need to be designed to withstand the high temperatures and pressures generated during the reaction. The waste generated during the reaction, such as the unreacted phenol and the acid washings, needs to be properly treated and disposed of to prevent environmental pollution.
White paper on Phenols Sulphonation
White Paper: Phenols Sulphonation – An Overview
Phenols sulphonation is a chemical process that involves the addition of a sulphonate group (-SO3H) to the aromatic ring of a phenol compound. Sulphonation is a key step in the production of various industrial chemicals, such as dyes, pigments, and pharmaceuticals. In this white paper, we will provide an overview of the phenols sulphonation process, including the mechanism, reaction conditions, and applications.
Mechanism of Phenols Sulphonation:
The sulphonation of phenols occurs via an electrophilic aromatic substitution reaction. The sulphonate group is generated in situ by the reaction of concentrated sulphuric acid with water. The sulphonation reaction occurs at the ortho-, meta-, or para-positions of the phenolic ring, depending on the reaction conditions and the structure of the phenol compound. The sulphonation reaction is highly exothermic and needs to be carefully controlled to prevent overheating and decomposition of the reactants.
Reaction Conditions:
The reaction conditions for phenols sulphonation depend on the type of phenol compound and the desired sulphonation product. Typically, concentrated sulphuric acid (H2SO4) is added to a mixture of the phenol compound and water in a reaction vessel. The mixture is then heated to a temperature of 80-100°C with constant stirring. Fuming sulphuric acid (oleum, H2S2O7) is then added dropwise to the reaction mixture to generate the sulphonate group. The reaction mixture is allowed to cool to room temperature, and the resulting mixture is neutralized with a base, such as sodium hydroxide (NaOH), to form the sulphonated product. The reaction conditions, including the temperature, reaction time, and concentration of the reagents, need to be optimized to achieve high yield and purity of the sulphonated product.
Applications:
Phenols sulphonation is used in the production of various industrial chemicals, including dyes, pigments, and pharmaceuticals. Sulphonated phenols are important intermediates in the synthesis of acid dyes, reactive dyes, and direct dyes. Phenols sulphonation is also used in the production of phenolic resins, which are used as adhesives and binders in the wood and paper industries. Sulphonated phenols are also used as stabilizers and surfactants in the production of polymers and detergents.
Environmental and Safety Considerations:
Phenols sulphonation is a hazardous chemical process that requires strict safety precautions to prevent accidents and environmental pollution. The concentrated sulphuric acid and fuming sulphuric acid used in the reaction are highly corrosive and can cause severe burns if they come into contact with the skin or eyes. The reaction is also highly exothermic, and the temperature needs to be carefully controlled to prevent overheating and decomposition of the reactants. The reaction vessel and the cooling system need to be designed to withstand the high temperatures and pressures generated during the reaction. The waste generated during the reaction, such as the unreacted phenol and the acid washings, needs to be properly treated and disposed of to prevent environmental pollution.
Conclusion:
Phenols sulphonation is a key step in the production of various industrial chemicals, including dyes, pigments, and pharmaceuticals. The process involves the addition of a sulphonate group to the aromatic ring of a phenol compound via an electrophilic aromatic substitution reaction. The reaction conditions need to be carefully optimized to achieve high yield and purity of the sulphonated product. However, the process is hazardous and requires strict safety precautions to prevent accidents and environmental pollution.
