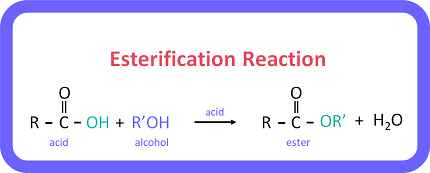
Esterification is a chemical reaction between an alcohol and a carboxylic acid in the presence of an acid catalyst to form an ester and water. The general equation for this reaction is:
R-OH + R’-COOH → R’-COOR + H2O
where R and R’ are alkyl or aryl groups.
There are several reactions that can take place during esterification, including:
- Condensation: The alcohol and carboxylic acid react to form water, which is then removed from the reaction mixture, driving the reaction towards the formation of the ester.
- Equilibrium: The reaction is reversible, and the equilibrium constant depends on the nature of the reactants, the temperature, and the catalyst used.
- Side reactions: Some side reactions, such as dehydration and the formation of other byproducts, can also occur during esterification.
Esterification is an important reaction in organic chemistry and is used in the synthesis of many organic compounds, including fragrances, flavors, and plastics. It is also used in the production of biodiesel, where vegetable oils or animal fats are esterified with methanol or ethanol to produce fatty acid esters that can be used as fuel.
What is Required Alcohols Reactions: Esterification
In the esterification reaction, any alcohol can be used as a reactant to form an ester. However, primary and secondary alcohols are the most commonly used alcohols in esterification reactions. Tertiary alcohols are not suitable for esterification because they do not have a reactive hydrogen atom attached to the carbon atom bearing the hydroxyl group.
The choice of alcohol used in the reaction can affect the properties of the resulting ester. For example, the length of the carbon chain in the alcohol can affect the volatility and solubility of the ester, while the presence of functional groups, such as double bonds or phenyl groups, can affect its reactivity and other physical and chemical properties.
In addition to the alcohol, a carboxylic acid and an acid catalyst are also required for the esterification reaction to occur. The acid catalyst is typically a mineral acid, such as sulfuric acid or hydrochloric acid, which helps to protonate the alcohol and promote the formation of the ester. Water is also produced as a byproduct of the reaction.
When is Required Alcohols Reactions: Esterification
Esterification reactions are commonly used in organic synthesis and industrial processes to produce esters. Esters have a wide range of applications, including as fragrances, flavors, solvents, plasticizers, and surfactants.
Esterification reactions are also used in the production of biodiesel, which involves the esterification of fatty acids in vegetable oils or animal fats with methanol or ethanol to produce fatty acid methyl or ethyl esters, respectively. These esters can be used as a renewable and sustainable alternative to fossil fuels.
In addition, esterification reactions are used in the synthesis of many organic compounds, such as pharmaceuticals, polymers, and resins. For example, aspirin is synthesized by the esterification of salicylic acid with acetic anhydride to produce acetylsalicylic acid.
Overall, esterification reactions are versatile and widely used in various fields of chemistry and industry.
Where is Required Alcohols Reactions: Esterification
Esterification reactions can be carried out in a variety of settings, including in a laboratory, pilot plant, or industrial scale.
In a laboratory, esterification reactions are typically carried out using small-scale glassware and standard laboratory equipment, such as round-bottom flasks, reflux condensers, and heating mantles. The reaction can be monitored using techniques such as thin-layer chromatography, infrared spectroscopy, or gas chromatography.
In a pilot plant or industrial setting, esterification reactions can be carried out on a larger scale using specialized equipment, such as reactors, distillation columns, and heat exchangers. The process is typically automated and controlled using computer systems to ensure reproducibility and consistency.
Esterification reactions can also be carried out in a continuous flow system, which offers several advantages over batch processes, such as improved efficiency, better control over reaction parameters, and reduced waste.
Overall, the location of esterification reactions depends on the specific application and scale of the process, ranging from a laboratory setting to an industrial production plant.
How is Required Alcohols Reactions: Esterification
Esterification reactions involve the conversion of an alcohol and a carboxylic acid into an ester and water. The reaction is typically catalyzed by an acid catalyst, such as sulfuric acid or hydrochloric acid. The mechanism of esterification involves several steps:
- Protonation: The acid catalyst protonates the alcohol, forming an oxonium ion.
- Nucleophilic attack: The carboxylic acid reacts with the oxonium ion, with the oxygen of the carboxylic acid acting as a nucleophile. This forms an intermediate molecule called an esterified acid.
- Proton transfer: The protonated acid catalyst transfers a proton to the esterified acid, forming the ester and regenerating the acid catalyst.
- Water removal: Water is formed as a byproduct of the reaction, which is typically removed by a Dean-Stark trap or azeotropic distillation to drive the reaction forward.
The overall reaction can be represented by the following equation:
R-OH + R’-COOH → R’-COOR + H2O
where R and R’ are alkyl or aryl groups.
The reaction conditions, including the temperature, concentration of reactants, and choice of acid catalyst, can be optimized to control the yield and selectivity of the reaction.
Nomenclature of Alcohols Reactions: Esterification
In the nomenclature of alcohols, the suffix “-ol” is used to indicate the presence of a hydroxyl group (-OH) attached to an alkyl or aryl group. For example, ethanol has the molecular formula C2H5OH, where “-ol” indicates the presence of a hydroxyl group.
In the nomenclature of esters produced by esterification reactions, the name of the parent carboxylic acid is used as the first part of the name, followed by the name of the alcohol with the “-yl” suffix replaced by “-ate”. For example, the ester formed from the reaction of ethyl alcohol (ethanol) with acetic acid is ethyl acetate, which has the chemical formula CH3COOCH2CH3.
In cases where the parent carboxylic acid has a common name, the ester name is derived from the common name. For example, the ester formed from the reaction of methanol and formic acid is commonly known as methyl formate.
Overall, the nomenclature of esters produced by esterification reactions follows a systematic naming convention based on the names of the parent carboxylic acid and alcohol.
Case Study on Alcohols Reactions: Esterification
One example of the use of esterification reactions in industry is in the production of biodiesel. Biodiesel is a renewable and sustainable alternative to fossil fuels that is derived from vegetable oils or animal fats. The production of biodiesel involves the esterification of fatty acids in the oils or fats with methanol or ethanol to produce fatty acid methyl or ethyl esters, respectively.
One case study of the esterification reaction in the production of biodiesel involves a large-scale plant in the United States. The plant produces over 100 million gallons of biodiesel per year, using soybean oil as the feedstock. The process involves several steps:
- Pretreatment: The soybean oil is treated to remove impurities, such as water, free fatty acids, and particulate matter. This step involves heating the oil to remove water and filtering it to remove solids.
- Transesterification: The pretreated soybean oil is mixed with methanol and an acid catalyst, such as sulfuric acid, to catalyze the esterification reaction. The mixture is heated and stirred to promote the reaction and remove water from the reaction mixture.
- Separation: The mixture is then allowed to settle, and the glycerol byproduct is removed from the bottom of the tank. The remaining liquid is washed with water to remove any remaining impurities.
- Drying: The washed biodiesel is then dried to remove any remaining water.
- Quality control: The biodiesel is tested to ensure that it meets the required specifications for use as a fuel.
Overall, the production of biodiesel using the esterification reaction is an example of the application of green chemistry principles, as it involves the use of renewable feedstocks and reduces dependence on fossil fuels. It also has the potential to reduce greenhouse gas emissions and promote sustainable development.
White paper on Alcohols Reactions: Esterification
Introduction:
Esterification is a chemical reaction that involves the formation of an ester from a carboxylic acid and an alcohol. This reaction is one of the most important organic reactions in the chemical industry, and it has a wide range of applications, including the production of biodiesel, fragrances, flavors, and pharmaceuticals. In this white paper, we will discuss the mechanisms, conditions, and applications of esterification reactions involving alcohols.
Mechanism:
The mechanism of esterification involves several steps. First, the alcohol is protonated by an acid catalyst, such as sulfuric acid or hydrochloric acid, to form an oxonium ion. Next, the carboxylic acid reacts with the oxonium ion, with the oxygen of the carboxylic acid acting as a nucleophile. This forms an intermediate molecule called an esterified acid. The protonated acid catalyst then transfers a proton to the esterified acid, forming the ester and regenerating the acid catalyst. Finally, water is removed from the reaction mixture to drive the reaction forward.
Conditions:
The conditions for esterification reactions depend on the nature of the reactants and the desired product. The reaction is typically catalyzed by an acid catalyst, and the concentration of the reactants can be optimized to control the yield and selectivity of the reaction. The temperature and pressure of the reaction can also be adjusted to promote the formation of the desired product. In some cases, azeotropic distillation or a Dean-Stark trap can be used to remove water from the reaction mixture.
Applications:
Esterification reactions involving alcohols have a wide range of applications in various industries. One of the most important applications is the production of biodiesel, which involves the esterification of fatty acids in vegetable oils or animal fats with methanol or ethanol. Esterification reactions are also used in the production of fragrances and flavors, such as esters of benzoic acid and alcohols. Additionally, esterification reactions are used in the pharmaceutical industry to synthesize ester prodrugs, which are compounds that are converted into active drugs in the body.
Conclusion:
Esterification reactions involving alcohols are important organic reactions with a wide range of applications in various industries. The mechanisms, conditions, and applications of esterification reactions can be optimized to produce high yields of the desired product. The use of esterification reactions can promote sustainable development by reducing dependence on fossil fuels and using renewable feedstocks.
