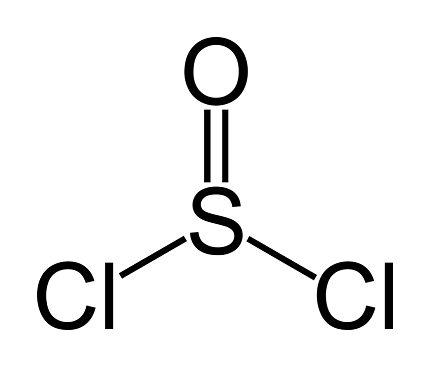
Thionyl chloride (SOCl2) is a chemical compound that is commonly used as a reagent in organic chemistry for various reactions, such as the conversion of alcohols to alkyl chlorides, carboxylic acids to acid chlorides, and amides to nitriles.
Thionyl chloride is a colorless to slightly yellowish liquid with a pungent odor. It is highly reactive and can be hazardous if not handled properly. It reacts violently with water, alcohols, and other protic solvents, releasing hydrogen chloride gas, which is corrosive and can cause severe irritation to the skin, eyes, and respiratory system.
Thionyl chloride is typically used under anhydrous conditions, and precautions should be taken to avoid contact with moisture. It is often used in a fume hood or with proper ventilation to prevent the inhalation of toxic fumes.
What is Required Alcohols Thionyl chloride
Thionyl chloride (SOCl2) is commonly used to convert alcohols to alkyl chlorides. The reaction involves substitution of the hydroxyl group (OH) of the alcohol with a chloride (Cl) group, resulting in the formation of an alkyl chloride.
The reaction typically requires an excess of thionyl chloride and is typically carried out under anhydrous conditions, as the reaction can be inhibited by the presence of water.
The reaction mechanism involves the formation of a chlorosulfite intermediate, which then reacts with a chloride ion to form the alkyl chloride product. The overall reaction can be represented as follows:
ROH + SOCl2 → RCl + SO2 + HCl
where R represents an alkyl group.
It is important to note that this reaction can be hazardous if not handled properly, and precautions should be taken to avoid contact with thionyl chloride and the resulting hydrogen chloride gas, which can be corrosive and toxic.
When is Required Alcohols Thionyl chloride
Thionyl chloride is commonly used in organic chemistry as a reagent to convert alcohols to alkyl chlorides. This reaction is often used in the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and other fine chemicals.
The conversion of alcohols to alkyl chlorides using thionyl chloride is an important reaction because alkyl chlorides are versatile intermediates that can be used for further chemical transformations. For example, alkyl chlorides can be used in nucleophilic substitution reactions to introduce a variety of functional groups, including amines, ethers, and carboxylic acids.
Thionyl chloride is particularly useful in this reaction because it is a relatively inexpensive and readily available reagent that is compatible with a wide range of alcohol substrates. Additionally, the reaction is usually carried out under mild conditions and does not require the use of harsh or expensive catalysts.
However, it is important to note that the use of thionyl chloride can be hazardous if not handled properly, and precautions should be taken to ensure that the reaction is carried out safely.
Where is Required Alcohols Thionyl chloride
The reaction of converting alcohols to alkyl chlorides using thionyl chloride is a widely used method in organic synthesis and can be performed in various laboratory settings. This reaction can be carried out on a small scale in a typical chemistry laboratory or on a larger scale in an industrial setting.
In the laboratory, the reaction is usually carried out in a flask or a reaction vessel under anhydrous conditions. The alcohol substrate is dissolved in a suitable solvent, such as dichloromethane or tetrahydrofuran, and then thionyl chloride is added dropwise to the mixture while stirring. The reaction is typically allowed to proceed for several hours at room temperature or under gentle heating.
In an industrial setting, the reaction is typically performed on a larger scale using specialized equipment designed for handling hazardous chemicals. The reaction may be carried out in a continuous flow process or in batches using large reaction vessels. Safety precautions, such as the use of protective clothing and specialized ventilation systems, are typically implemented to ensure that the reaction is carried out safely.
Overall, the reaction of converting alcohols to alkyl chlorides using thionyl chloride is a versatile and widely used method in organic synthesis that can be performed in various laboratory and industrial settings.
How is Required Alcohols Thionyl chloride
The conversion of alcohols to alkyl chlorides using thionyl chloride is a common reaction in organic chemistry. The reaction typically requires an excess of thionyl chloride and is typically carried out under anhydrous conditions, as the presence of water can inhibit the reaction.
The reaction mechanism involves the substitution of the hydroxyl group (OH) of the alcohol with a chloride (Cl) group, resulting in the formation of an alkyl chloride. The overall reaction can be represented as follows:
ROH + SOCl2 → RCl + SO2 + HCl
where R represents an alkyl group.
The reaction can be carried out in a variety of solvents, including dichloromethane, tetrahydrofuran, and chloroform. The alcohol substrate is typically dissolved in the solvent, and then an excess of thionyl chloride is added dropwise to the mixture while stirring.
The reaction is usually allowed to proceed for several hours at room temperature or under gentle heating. After the reaction is complete, the excess thionyl chloride and the byproduct hydrogen chloride gas are removed by evaporation under reduced pressure.
The resulting alkyl chloride product can be purified by distillation, column chromatography, or other methods, depending on the specific application.
It is important to note that the use of thionyl chloride can be hazardous if not handled properly, and precautions should be taken to ensure that the reaction is carried out safely. Appropriate protective equipment, such as gloves and goggles, should be worn, and the reaction should be carried out in a well-ventilated area.
Production of Alcohols Thionyl chloride
Thionyl chloride is not typically used in the production of alcohols, as it is primarily used to convert alcohols to alkyl chlorides. However, alcohols can be produced using a variety of methods, including fermentation, synthesis from other organic compounds, and hydrolysis of corresponding alkyl halides or alkyl sulfates.
Fermentation is a common method for the production of alcohols, particularly ethanol, which is produced by the fermentation of sugars by yeast. This method is widely used in the production of alcoholic beverages and biofuels.
Another method for the production of alcohols involves the synthesis of alcohols from other organic compounds, such as aldehydes and ketones. This can be accomplished using a variety of reagents, such as reducing agents like sodium borohydride or lithium aluminum hydride.
Hydrolysis of corresponding alkyl halides or alkyl sulfates is also a method for the production of alcohols. This method involves the use of a strong base, such as sodium hydroxide or potassium hydroxide, to cleave the halide or sulfate group from the alkyl compound, resulting in the formation of an alcohol.
Overall, thionyl chloride is not typically used in the production of alcohols, but rather as a reagent for converting alcohols to alkyl chlorides.
Case Study on Alcohols Thionyl chloride
One example of the use of thionyl chloride in the synthesis of organic compounds involves the conversion of allylic alcohols to allylic chlorides. Allylic chlorides are useful intermediates in the synthesis of a variety of organic compounds, including pharmaceuticals and agrochemicals.
In a study published in the journal “Organic Letters,” researchers demonstrated the use of thionyl chloride in the conversion of allylic alcohols to allylic chlorides. The reaction was carried out under mild conditions, and the resulting allylic chlorides were obtained in high yields.
The researchers began by selecting a range of allylic alcohols with different substituents, including methyl, ethyl, and phenyl groups. The alcohols were dissolved in dichloromethane, and thionyl chloride was added dropwise to the mixture while stirring.
The reaction was allowed to proceed for several hours at room temperature, and the progress of the reaction was monitored by thin-layer chromatography. After the reaction was complete, the excess thionyl chloride was removed by evaporation under reduced pressure.
The resulting allylic chlorides were purified by column chromatography and characterized by nuclear magnetic resonance spectroscopy. The researchers found that the reaction was successful in converting a range of allylic alcohols to allylic chlorides, with high yields and good selectivity.
Overall, this study demonstrates the utility of thionyl chloride as a reagent for the conversion of allylic alcohols to allylic chlorides, highlighting its importance in the synthesis of organic compounds with diverse applications.
White paper on Alcohols Thionyl chloride
Introduction:
Thionyl chloride is a versatile reagent that is widely used in organic chemistry for the conversion of alcohols to alkyl chlorides. This reaction has been studied extensively and is an important tool for the synthesis of a variety of organic compounds. In this white paper, we will discuss the properties of thionyl chloride, its use in the conversion of alcohols to alkyl chlorides, and its potential applications in organic synthesis.
Properties of Thionyl Chloride:
Thionyl chloride is a colorless, fuming liquid that is soluble in a variety of organic solvents. It has a boiling point of 79.4 °C and a density of 1.63 g/cm³. Thionyl chloride is a strong electrophile, which makes it an effective reagent for the conversion of alcohols to alkyl chlorides. It reacts with alcohols to form alkyl chlorides and produces hydrogen chloride gas as a byproduct.
Conversion of Alcohols to Alkyl Chlorides:
The conversion of alcohols to alkyl chlorides using thionyl chloride is a well-established reaction in organic chemistry. This reaction is typically carried out under anhydrous conditions to prevent the formation of oxides or other byproducts. The reaction involves the substitution of the hydroxyl group (OH) of the alcohol with a chloride (Cl) group, resulting in the formation of an alkyl chloride. The overall reaction can be represented as follows:
ROH + SOCl2 → RCl + SO2 + HCl
where R represents an alkyl group.
The reaction can be carried out in a variety of solvents, including dichloromethane, tetrahydrofuran, and chloroform. The alcohol substrate is typically dissolved in the solvent, and then an excess of thionyl chloride is added dropwise to the mixture while stirring. The reaction is usually allowed to proceed for several hours at room temperature or under gentle heating. After the reaction is complete, the excess thionyl chloride and the byproduct hydrogen chloride gas are removed by evaporation under reduced pressure.
Potential Applications in Organic Synthesis:
Thionyl chloride has a wide range of potential applications in organic synthesis. In addition to its use in the conversion of alcohols to alkyl chlorides, it can be used in the synthesis of acid chlorides, isocyanates, and other reactive intermediates. Thionyl chloride is also an effective reagent for the dehydration of primary amides to nitriles, the conversion of carboxylic acids to acid chlorides, and the synthesis of phosphorus oxychloride.
Conclusion:
Thionyl chloride is a powerful reagent that has a wide range of potential applications in organic synthesis. Its ability to convert alcohols to alkyl chlorides makes it a valuable tool for the synthesis of a variety of organic compounds, including pharmaceuticals, agrochemicals, and specialty chemicals. However, it is important to use thionyl chloride with caution, as it can be hazardous if not handled properly. Appropriate protective equipment should be worn, and the reaction should be carried out in a well-ventilated area.
