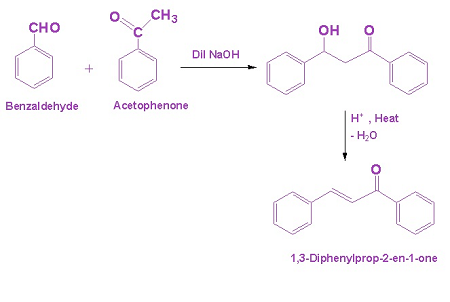
Aldol condensation is a type of organic reaction that involves the condensation of two carbonyl compounds, usually an aldehyde and a ketone, to form a β-hydroxy carbonyl compound, also known as an aldol. The word “aldol” is a combination of “aldehyde” and “alcohol”.
The reaction typically requires a base catalyst, such as sodium hydroxide, to deprotonate the carbonyl compound and form an enolate ion, which then attacks the carbonyl carbon of the other molecule. The resulting intermediate undergoes protonation to yield the final product.
The aldol condensation can be classified into two types: the crossed aldol condensation, in which the two carbonyl compounds are different, and the self-aldol condensation, in which the two carbonyl compounds are identical.
The aldol condensation is an important reaction in organic synthesis, as it allows for the formation of new carbon-carbon bonds, which are essential for the synthesis of many organic compounds. The reaction can also be used to create a variety of functional groups, such as α,β-unsaturated carbonyl compounds and cyclohexenones.
What is Required Aldehydes and Ketones Aldol Condensation
The Aldol condensation is a reaction that requires two types of carbonyl compounds: an aldehyde and a ketone.
The aldehyde has a terminal carbonyl group (-CHO) and is typically represented by the general formula RCHO, where R is a substituent group. The aldehyde is usually the more reactive of the two carbonyl compounds and serves as the nucleophile in the reaction.
The ketone, on the other hand, has an internal carbonyl group (>C=O) and is typically represented by the general formula RCOR’, where R and R’ are substituent groups. The ketone serves as the electrophile in the reaction.
Both the aldehyde and ketone must have at least one α-hydrogen, which is necessary for the formation of the enolate intermediate. The α-hydrogen is the hydrogen atom attached to the carbon adjacent to the carbonyl group.
Overall, the Aldol condensation is a powerful tool for the formation of new carbon-carbon bonds and is widely used in organic synthesis to create complex molecules with multiple functional groups.
When is Required Aldehydes and Ketones Aldol Condensation
The Aldol condensation is commonly used in organic synthesis to form new carbon-carbon bonds between two carbonyl compounds, typically an aldehyde and a ketone. This reaction is particularly useful for creating complex molecules with multiple functional groups, as it allows for the formation of a β-hydroxy carbonyl compound, also known as an aldol.
The Aldol condensation reaction can be used in a variety of situations, such as:
- Synthesis of natural products: The Aldol condensation is commonly used in the synthesis of natural products such as steroids, terpenes, and alkaloids.
- Drug discovery: The Aldol condensation can be used to synthesize new drug molecules by creating specific structural motifs and functional groups.
- Material science: The Aldol condensation can be used to create polymers, resins, and other materials with unique properties.
- Organic chemistry research: The Aldol condensation is a fundamental reaction in organic chemistry and is often used in research to study reaction mechanisms and develop new synthetic strategies.
Overall, the Aldol condensation is a versatile and powerful tool for the formation of new carbon-carbon bonds and is widely used in organic synthesis and research.
Where is Required Aldehydes and Ketones Aldol Condensation
The Aldol condensation can be performed in a variety of settings, including in a laboratory or industrial setting.
In a laboratory setting, the Aldol condensation can be carried out using basic glassware such as a round-bottom flask, a magnetic stir bar, and a condenser. The reaction is typically performed under reflux conditions with a basic catalyst such as sodium hydroxide or potassium hydroxide.
In an industrial setting, the Aldol condensation can be carried out using specialized equipment designed for large-scale chemical reactions. The reaction is typically carried out in a continuous-flow system, which allows for the efficient production of large quantities of product.
The Aldol condensation is a widely used reaction in organic synthesis, and as such, can be found in many chemical industries, including pharmaceuticals, agrochemicals, and materials science.
Overall, the Aldol condensation can be performed in a variety of settings depending on the scale of the reaction and the desired product output.
How is Required Aldehydes and Ketones Aldol Condensation
The Aldol condensation reaction involves the reaction between an aldehyde and a ketone to form a β-hydroxy carbonyl compound, also known as an aldol. The reaction is typically catalyzed by a base, such as sodium hydroxide or potassium hydroxide, and occurs in several steps:
- Enolate Formation: The aldehyde or ketone is deprotonated by the base to form an enolate ion. The enolate ion is a nucleophile that is capable of attacking the carbonyl carbon of the other carbonyl compound.
- Addition: The enolate ion attacks the carbonyl carbon of the other carbonyl compound, forming a carbon-carbon bond. This creates an intermediate that contains an α,β-unsaturated carbonyl compound.
- Protonation: The intermediate is then protonated by the base or by an acid to form the aldol product. The aldol product is a β-hydroxy carbonyl compound that contains a hydroxyl group and an aldehyde or ketone functional group.
The reaction can also proceed through a crossed aldol condensation, where the two carbonyl compounds are different, or a self-aldol condensation, where the two carbonyl compounds are identical.
Overall, the Aldol condensation is a versatile reaction that allows for the formation of new carbon-carbon bonds and is widely used in organic synthesis to create complex molecules with multiple functional groups.
Production of Aldehydes and Ketones Aldol Condensation
Aldehydes and ketones can be produced by a variety of methods, including oxidation of primary and secondary alcohols, respectively. Once the aldehydes and ketones are obtained, they can then be used in the Aldol condensation reaction to form β-hydroxy carbonyl compounds.
Here are some common methods for the production of aldehydes and ketones:
- Oxidation of primary alcohols: Primary alcohols can be oxidized to aldehydes using oxidizing agents such as pyridinium chlorochromate (PCC) or Jones reagent (a mixture of chromic acid and sulfuric acid). The reaction is typically carried out under mild conditions to prevent further oxidation of the aldehyde to a carboxylic acid.
- Oxidation of secondary alcohols: Secondary alcohols can be oxidized to ketones using oxidizing agents such as chromic acid or potassium permanganate. The reaction is typically carried out under more vigorous conditions than the oxidation of primary alcohols.
- Friedel-Crafts Acylation: Aromatic ketones can be synthesized by the Friedel-Crafts acylation reaction, which involves the reaction of an aromatic compound with an acid chloride in the presence of a Lewis acid catalyst, such as aluminum chloride.
- Wittig Reaction: Aldehydes and ketones can also be synthesized using the Wittig reaction, which involves the reaction of an aldehyde or ketone with a phosphonium ylide to form an alkene and a phosphine oxide.
Overall, there are several methods for the production of aldehydes and ketones, which can then be used in the Aldol condensation reaction to form β-hydroxy carbonyl compounds.
Case Study on Aldehydes and Ketones Aldol Condensation
One example of the application of the Aldol condensation reaction is in the synthesis of the anti-inflammatory drug naproxen. Naproxen is a non-steroidal anti-inflammatory drug (NSAID) that is commonly used to treat pain and inflammation.
The synthesis of naproxen involves several steps, including the Aldol condensation reaction. Here is a brief overview of the synthesis:
- Synthesis of the key intermediate: The synthesis begins with the synthesis of a key intermediate, 2-(6-methoxy-2-naphthyl)propionic acid, which is obtained through a series of steps involving the Grignard reaction, acid hydrolysis, and decarboxylation.
- Aldol condensation: The key intermediate is then subjected to an Aldol condensation reaction with acetic anhydride in the presence of sodium acetate as a catalyst. The Aldol product is then dehydrated to form an α,β-unsaturated ketone.
- Reduction: The α,β-unsaturated ketone is then reduced using a reducing agent, such as lithium aluminum hydride, to form the naproxen intermediate.
- Resolution: The naproxen intermediate is then resolved into its two enantiomers using a chiral acid, such as D-tartaric acid.
- Esterification: Finally, the resolved enantiomers are esterified with methanol to form the corresponding methyl esters, which are then hydrolyzed to form the final product, naproxen.
Overall, the Aldol condensation reaction plays a key role in the synthesis of naproxen, allowing for the formation of a new carbon-carbon bond and the creation of the α,β-unsaturated ketone intermediate. The synthesis of naproxen is a complex and multi-step process, but demonstrates the importance of the Aldol condensation reaction in the synthesis of complex molecules.
White paper on Aldehydes and Ketones Aldol Condensation
Introduction:
Aldehydes and ketones are important organic compounds that find a variety of applications in industry, medicine, and research. One important reaction involving these compounds is the Aldol condensation reaction, which allows for the formation of new carbon-carbon bonds and the synthesis of more complex molecules.
Mechanism:
The Aldol condensation reaction involves the reaction of an aldehyde or ketone with a carbonyl compound under basic conditions to form a β-hydroxy carbonyl compound. The reaction proceeds through an enolate intermediate, which is formed by deprotonation of the carbonyl compound in the presence of a base. The enolate then attacks the carbonyl carbon of the aldehyde or ketone, forming a new carbon-carbon bond and a β-hydroxy carbonyl compound.
The β-hydroxy carbonyl compound can then undergo further reactions, such as dehydration, to form an α,β-unsaturated carbonyl compound. The Aldol condensation reaction is therefore a versatile reaction that can be used to synthesize a variety of compounds, including α,β-unsaturated ketones and aldehydes.
Applications:
The Aldol condensation reaction has many applications in organic synthesis. One important application is in the synthesis of natural products and pharmaceuticals. For example, the synthesis of the anti-inflammatory drug naproxen involves an Aldol condensation reaction as a key step.
In addition, the Aldol condensation reaction can be used to synthesize polymers, such as polyesters and polyamides. The reaction can also be used in the synthesis of materials, such as resins and adhesives, that have a variety of industrial applications.
Conclusion:
The Aldol condensation reaction is a versatile and important reaction in organic synthesis, allowing for the formation of new carbon-carbon bonds and the synthesis of more complex molecules. The reaction has many applications in industry, medicine, and research, and continues to be an area of active investigation in the field of organic chemistry.
