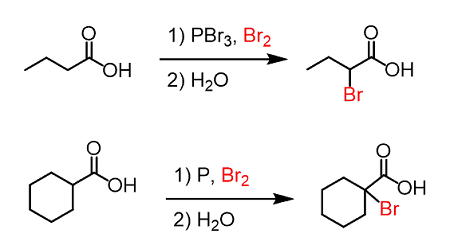
Carboxylic acids are not usually halogenated directly as they are not very reactive towards halogens. However, there are a few methods for the halogenation of carboxylic acids. One method involves the use of phosphorus halides, such as phosphorus tribromide (PBr3) or phosphorus pentachloride (PCl5), to convert the carboxylic acid into an acyl halide.
The reaction can be represented as follows:
RCOOH + PBr3 → RCOBr + HBr + P(OH)3
or
RCOOH + PCl5 → RCOCl + HCl + POCl3
In this reaction, the phosphorus halide reacts with the carboxylic acid to form an intermediate which then reacts with the water present in the reaction mixture to form the acyl halide and the corresponding acid.
Another method for halogenating carboxylic acids involves the use of N-halosuccinimide (NHS) or N-bromosuccinimide (NBS). These reagents react with the carboxylic acid to form an intermediate, which then reacts with the halogen to form the corresponding halogenated carboxylic acid.
The reaction can be represented as follows:
RCOOH + NXS → RCOX + HX + NSO2X (X = halogen)
In this reaction, the NXS reagent reacts with the carboxylic acid to form an intermediate, which then reacts with the halogen to form the halogenated carboxylic acid and N-halosuccinimide or N-bromosuccinimide.
Overall, halogenation of carboxylic acids is possible through the use of specific reagents, but it is generally not a common transformation due to the low reactivity of carboxylic acids towards halogens.
What is Required Carboxylic Acids Halogenation
Halogenation of carboxylic acids typically requires a halogenating reagent, such as phosphorus tribromide (PBr3) or phosphorus pentachloride (PCl5), or N-halosuccinimide (NHS) or N-bromosuccinimide (NBS). These reagents are used to convert the carboxylic acid into an acyl halide or to form an intermediate that can react with the halogen to form the halogenated carboxylic acid.
The reaction may also require appropriate solvents and reaction conditions, such as appropriate temperature and stirring, to facilitate the reaction and achieve a good yield of the desired product.
It is also important to note that halogenation of carboxylic acids can result in side reactions, such as formation of the corresponding acid and hydrogen halide or formation of byproducts, which can affect the yield and purity of the desired product. Therefore, proper purification methods may also be required to obtain the desired product in a pure form.
When is Required Carboxylic Acids Halogenation
Carboxylic acid halogenation may be required in various organic synthesis applications. Halogenated carboxylic acids and their derivatives can be used as important building blocks in the synthesis of a wide range of organic compounds, including pharmaceuticals, agrochemicals, and natural products.
For example, halogenated carboxylic acids can be used as intermediates in the synthesis of amides, esters, and other functional derivatives. In addition, halogenated carboxylic acids can also be used as starting materials in the synthesis of carboxylic acid derivatives, such as acid chlorides, anhydrides, and esters.
Halogenation of carboxylic acids may also be used in the preparation of functionalized materials, such as polymers, and in the modification of natural products for improved activity or stability.
Overall, carboxylic acid halogenation can be a useful tool in organic synthesis, providing a way to introduce halogen atoms into carboxylic acid molecules and generate new functional groups for further modification and derivatization.
Where is Required Carboxylic Acids Halogenation
Carboxylic acid halogenation can be used in various fields where organic synthesis is important. Some of the areas where carboxylic acid halogenation may be required include:
- Pharmaceutical industry: Halogenated carboxylic acids can be used as building blocks for the synthesis of pharmaceuticals, such as antibiotics, antivirals, and anticancer drugs.
- Agrochemical industry: Halogenated carboxylic acids can also be used in the synthesis of agrochemicals, such as herbicides, fungicides, and insecticides.
- Material science: Halogenated carboxylic acids can be used as building blocks in the synthesis of functionalized polymers and materials, such as sensors, electronic devices, and bioactive materials.
- Natural product modification: Halogenation of carboxylic acids can be used in the modification of natural products to improve their biological activity or stability.
- Chemical research: Carboxylic acid halogenation is a useful tool in chemical research for generating new compounds and studying their properties.
Overall, carboxylic acid halogenation has applications in various fields where organic synthesis plays an important role, and can be used to generate new compounds with diverse applications.
How is Required Carboxylic Acids Halogenation
The method for halogenation of carboxylic acids depends on the specific reagents and conditions used. Two common methods for carboxylic acid halogenation are:
- Halogenation using phosphorus halides: In this method, a carboxylic acid is reacted with a phosphorus halide, such as phosphorus tribromide (PBr3) or phosphorus pentachloride (PCl5), to form an acyl halide. The reaction is typically carried out in an anhydrous solvent, such as dichloromethane or chloroform, under reflux conditions. The resulting acyl halide can be isolated by distillation or extraction.
- Halogenation using N-halosuccinimide (NHS) or N-bromosuccinimide (NBS): In this method, a carboxylic acid is reacted with N-halosuccinimide (NHS) or N-bromosuccinimide (NBS) to form an intermediate. The intermediate then reacts with the halogen to form the halogenated carboxylic acid. The reaction is typically carried out in an inert solvent, such as dichloromethane or chloroform, under reflux conditions.
The choice of the method depends on factors such as the desired product, the availability of reagents, and the reaction conditions required. It is important to note that carboxylic acid halogenation can result in side reactions, such as formation of the corresponding acid and hydrogen halide or formation of byproducts, which can affect the yield and purity of the desired product. Therefore, proper purification methods may also be required to obtain the desired product in a pure form.
Production of Carboxylic Acids Halogenation
Carboxylic acid halogenation can be produced in a variety of ways, depending on the specific carboxylic acid and the desired halogenation reaction. Here are some general steps that may be involved in the production of halogenated carboxylic acids:
- Selection of the carboxylic acid: The first step is to select the appropriate carboxylic acid that will be used for the halogenation reaction. The carboxylic acid may be obtained from natural sources, synthesized through organic reactions, or purchased from chemical suppliers.
- Preparation of the reaction mixture: Once the carboxylic acid has been selected, it is dissolved in a suitable solvent, such as dichloromethane, chloroform, or a mixture of solvents, depending on the specific reaction. The halogenating reagent, such as phosphorus tribromide (PBr3), phosphorus pentachloride (PCl5), or N-halosuccinimide (NHS), is then added to the reaction mixture.
- Halogenation reaction: The reaction mixture is heated under reflux conditions or at a specific temperature range, depending on the reaction conditions required for the specific halogenating reagent. During the reaction, the carboxylic acid is transformed into an acyl halide or an intermediate that reacts with the halogenating reagent to form the halogenated carboxylic acid.
- Work-up and purification: After the reaction is complete, the reaction mixture is quenched with water or a quenching agent to remove excess halogenating reagent and reaction byproducts. The resulting mixture is then extracted with an appropriate solvent and washed with water to remove any remaining impurities. The halogenated carboxylic acid is then isolated by evaporation, distillation, or chromatography.
Overall, the production of halogenated carboxylic acids requires careful selection of the carboxylic acid, the halogenating reagent, and the reaction conditions, as well as proper work-up and purification methods to obtain the desired product in a pure form.
Case Study on Carboxylic Acids Halogenation
One example of a case study involving carboxylic acid halogenation is the synthesis of 2,3,4-trihydroxybenzophenone-5-carboxylic acid (THBPCA), which is an intermediate used in the synthesis of a class of antibacterial compounds called arylomycin antibiotics.
In this case study, THBPCA was synthesized using a multi-step process that involved carboxylic acid halogenation as one of the key steps. The synthesis involved the following steps:
- Synthesis of 2,4,6-trimethoxybenzoic acid: This compound was synthesized from commercially available materials through a series of reactions that involved esterification, hydrolysis, and decarboxylation.
- Synthesis of 2,3,4-trimethoxybenzophenone: This intermediate was synthesized by Friedel-Crafts acylation of 2,4,6-trimethoxybenzoic acid using acetyl chloride as the acylating agent.
- Synthesis of 2,3,4-trihydroxybenzophenone-5-carboxylic acid: This compound was synthesized by first converting 2,3,4-trimethoxybenzophenone to the corresponding carboxylic acid using potassium permanganate as the oxidizing agent. The resulting carboxylic acid was then halogenated using phosphorus tribromide (PBr3) to form the corresponding acyl bromide. Finally, the acyl bromide was hydrolyzed using sodium hydroxide to yield 2,3,4-trihydroxybenzophenone-5-carboxylic acid.
The carboxylic acid halogenation step was crucial for the synthesis of THBPCA, as it enabled the conversion of the carboxylic acid to the corresponding acyl bromide, which was then used in the subsequent hydrolysis reaction to yield the desired product. The choice of phosphorus tribromide as the halogenating reagent was based on its ability to selectively halogenate the carboxylic acid, while minimizing side reactions.
Overall, this case study highlights the importance of carboxylic acid halogenation as a key step in the synthesis of complex organic compounds, and demonstrates how it can be used in conjunction with other synthetic methods to achieve the desired product.
White paper on Carboxylic Acids Halogenation
Introduction:
Carboxylic acid halogenation is a versatile and widely used synthetic method in organic chemistry that enables the conversion of carboxylic acids to their corresponding acyl halides. This reaction is important in many areas of chemistry, including pharmaceuticals, materials science, and organic synthesis. In this white paper, we will provide an overview of carboxylic acid halogenation, including its mechanism, applications, and current research.
Mechanism:
The mechanism of carboxylic acid halogenation involves the reaction of the carboxylic acid with a halogenating reagent, such as phosphorus tribromide (PBr3), phosphorus pentachloride (PCl5), or N-halosuccinimide (NHS). The halogenating reagent reacts with the carboxylic acid to form an acyl halide intermediate, which can then undergo further reactions, such as nucleophilic substitution, to yield a variety of functionalized products. The overall reaction can be represented as follows:
Carboxylic acid + Halogenating reagent → Acyl halide intermediate + Byproducts
Applications:
Carboxylic acid halogenation has numerous applications in synthetic organic chemistry. Some of the important applications include:
- Synthesis of pharmaceuticals: Carboxylic acid halogenation is an important step in the synthesis of many pharmaceuticals, including arylomycin antibiotics, anti-inflammatory agents, and antipsychotic drugs.
- Materials science: Carboxylic acid halogenation is used in the synthesis of materials such as liquid crystals, polymers, and dyes.
- Organic synthesis: Carboxylic acid halogenation is a useful method for the introduction of functional groups into organic molecules. It can be used to synthesize a wide range of compounds, including alcohols, amines, and esters.
Current Research:
Recent research in carboxylic acid halogenation has focused on the development of new halogenating reagents and the optimization of reaction conditions. For example, researchers have developed new halogenating reagents that are more selective and efficient than traditional reagents, allowing for the synthesis of more complex molecules. Additionally, researchers have explored the use of flow chemistry and other advanced synthetic methods to optimize the reaction conditions and improve the scalability of carboxylic acid halogenation.
Conclusion:
Carboxylic acid halogenation is an important synthetic method in organic chemistry that has numerous applications in pharmaceuticals, materials science, and organic synthesis. The reaction mechanism is well understood, and recent research has focused on the development of new reagents and the optimization of reaction conditions. Overall, carboxylic acid halogenation is a versatile and valuable tool in the synthetic chemist’s toolkit.
