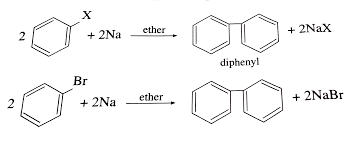
Fittig is a reaction in organic chemistry named after the German chemist Friedrich Fittig. The Fittig reaction involves the coupling of two aryl or vinyl halides to form a biaryl or bivinyl compound, respectively, in the presence of a metallic sodium or potassium. The reaction is carried out in an inert solvent such as ether or benzene.
The Fittig reaction is a useful method for the synthesis of symmetric and unsymmetric biaryls and bivinyls, which have applications in materials science, medicinal chemistry, and natural product synthesis. However, the reaction is limited by the availability and reactivity of the starting materials, and the need for careful control of the reaction conditions to prevent side reactions and decomposition.
Overall, the Fittig reaction is a powerful tool in the synthetic chemist’s toolbox, and has contributed to the development of many important compounds and materials.
What is Required Haloarenes Reactions: Fittig
The Fittig reaction involves the coupling of two aryl or vinyl halides to form a biaryl or bivinyl compound, respectively. Therefore, the required reactants for the Fittig reaction are two haloarenes, one aryl or vinyl halide as the electrophile, and one alkyl halide (usually sodium or potassium) as the nucleophile. The reaction also requires an inert solvent such as ether or benzene to facilitate the reaction.
The general reaction scheme for the Fittig reaction is:
2 Ar-X + 2 Na -> Ar-Ar + 2 NaX
where Ar represents an aryl or vinyl group and X represents a halogen atom (e.g., Cl, Br, I).
The Fittig reaction can be used to synthesize a wide range of biaryl and bivinyl compounds with various substitution patterns and functional groups. However, the reaction can be limited by the availability and reactivity of the starting materials, and the need for careful control of the reaction conditions to prevent side reactions and decomposition.
When is Required Haloarenes Reactions: Fittig
The Fittig reaction is commonly used in organic synthesis when a biaryl or bivinyl compound is needed. Biaryls and bivinyls are important classes of compounds that have a wide range of applications in materials science, medicinal chemistry, and natural product synthesis.
The Fittig reaction can be used to synthesize a variety of biaryls and bivinyls with different substitution patterns and functional groups. For example, the reaction can be used to synthesize biaryls and bivinyls that contain electron-donating or electron-withdrawing groups, which can affect the electronic properties of the resulting compounds.
The Fittig reaction can also be used to synthesize complex biaryls and bivinyls that are difficult to obtain by other methods. For example, the reaction can be used to synthesize biaryls and bivinyls that contain heteroatoms, such as nitrogen or sulfur, in the aromatic rings.
Overall, the Fittig reaction is a versatile and widely used method for the synthesis of biaryls and bivinyls, and is an important tool in the synthetic chemist’s toolbox.
Where is Required Haloarenes Reactions: Fittig
The Fittig reaction can be carried out in the laboratory using standard glassware and equipment. The reaction is typically performed in an inert solvent, such as ether or benzene, in the presence of a metallic sodium or potassium. The haloarene reactants are dissolved in the solvent and the metallic alkyl halide is added slowly with stirring under an inert atmosphere, such as nitrogen or argon.
The Fittig reaction can be used to synthesize a variety of biaryl and bivinyl compounds in the laboratory setting, and has applications in various fields of organic synthesis, such as materials science, medicinal chemistry, and natural product synthesis.
The reaction conditions, such as the choice of solvent, the reactivity of the haloarenes and the metallic alkyl halide, and the temperature, can be carefully controlled to optimize the yield and selectivity of the reaction. Overall, the Fittig reaction is a useful and versatile tool for the synthesis of biaryl and bivinyl compounds in the laboratory setting.
How is Required Haloarenes Reactions: Fittig
The Fittig reaction involves the coupling of two aryl or vinyl halides to form a biaryl or bivinyl compound, respectively, in the presence of metallic sodium or potassium. The reaction can be carried out as follows:
- Dissolve the two aryl or vinyl halides in an inert solvent, such as ether or benzene.
- Add a small amount of metallic sodium or potassium to the reaction mixture, which generates an alkyl halide intermediate.
- The alkyl halide intermediate then reacts with the aryl or vinyl halide to form the desired biaryl or bivinyl compound.
- The reaction mixture is then quenched with water or another suitable reagent to stop the reaction.
The reaction conditions, such as the choice of solvent, the reactivity of the haloarenes and the metallic alkyl halide, and the temperature, can be carefully controlled to optimize the yield and selectivity of the reaction. Side reactions, such as elimination or disproportionation, may also occur and can be minimized by careful control of the reaction conditions.
The Fittig reaction is a useful method for the synthesis of symmetric and unsymmetric biaryls and bivinyls, which have applications in materials science, medicinal chemistry, and natural product synthesis. However, the reaction is limited by the availability and reactivity of the starting materials, and the need for careful control of the reaction conditions to prevent side reactions and decomposition.
Production of Haloarenes Reactions: Fittig
The Fittig reaction is a useful method for the synthesis of biaryl and bivinyl compounds, including haloarenes, which are commonly used as starting materials in various organic syntheses. However, the Fittig reaction is not typically used for the production of haloarenes, as the reaction typically involves the coupling of two haloarenes to form a biaryl compound.
Instead, haloarenes are typically produced by other methods, such as halogenation reactions or by nucleophilic aromatic substitution reactions. For example, chlorobenzene can be synthesized by the chlorination of benzene using a halogenating agent such as chlorine gas or iron(III) chloride as a catalyst. Similarly, bromobenzene can be produced by the bromination of benzene using a brominating agent such as bromine or N-bromosuccinimide (NBS).
Once produced, haloarenes can then be used as starting materials in various organic syntheses, including the Fittig reaction, to form biaryl or bivinyl compounds. The Fittig reaction can be used to synthesize a variety of biaryl and bivinyl compounds with different substitution patterns and functional groups, and is a useful tool in the synthetic chemist’s toolbox.
Case Study on Haloarenes Reactions: Fittig
One example of the use of the Fittig reaction in organic synthesis involves the synthesis of 1,1′-bi-2-naphthol (BINOL), a chiral molecule with numerous applications in organic synthesis and materials science. BINOL is often used as a chiral ligand in asymmetric catalysis, and its derivatives have been used in the synthesis of natural products, pharmaceuticals, and materials.
The Fittig reaction has been used as a key step in the synthesis of BINOL by coupling two naphthyl halides to form a biphenyl compound, which is then oxidized to form BINOL. The reaction proceeds as follows:
- 2-naphthol is converted to 2-naphthyl chloride using thionyl chloride.
- 2-naphthyl chloride is coupled with 1-naphthyl chloride using metallic sodium in dry ether to form 1,1′-binaphthyl.
- The biphenyl compound is then oxidized using an oxidizing agent, such as chromium trioxide, to form the desired BINOL.
The Fittig reaction step in this synthesis allows for the formation of the biphenyl intermediate in good yield and selectivity, which can then be further transformed into BINOL. The reaction conditions, such as the choice of solvent, the reactivity of the naphthyl halides, and the temperature, are carefully controlled to optimize the yield and selectivity of the reaction.
Overall, the Fittig reaction plays an important role in the synthesis of BINOL and its derivatives, which have numerous applications in organic synthesis and materials science.
White paper on Haloarenes Reactions: Fittig
Introduction
The Fittig reaction, also known as the Wurtz-Fittig reaction, is a well-known organic synthesis reaction that involves the coupling of two aryl or vinyl halides to form a biaryl or bivinyl compound, respectively. The reaction is typically carried out in the presence of metallic sodium or potassium in an inert solvent, such as ether or benzene. The Fittig reaction is a useful tool in the synthetic chemist’s toolbox, as it allows for the synthesis of a variety of biaryl and bivinyl compounds with different substitution patterns and functional groups. In this white paper, we will discuss the Fittig reaction in more detail, including its mechanism, scope, and applications.
Mechanism of the Fittig Reaction
The Fittig reaction proceeds via a radical mechanism, where the metallic sodium or potassium serves as a reducing agent to generate an alkyl halide intermediate. The alkyl halide intermediate then reacts with the aryl or vinyl halide to form the desired biaryl or bivinyl compound. The overall reaction can be represented as follows:
ArX + R-X + 2Na → Ar-R + 2NaX
where Ar represents an aryl or vinyl group, X represents a halogen atom, R represents an alkyl group, and Na represents metallic sodium.
The reaction mechanism involves several steps, including the formation of the alkyl halide intermediate, the formation of the radical species, and the coupling of the two radicals to form the desired biaryl or bivinyl compound. The exact mechanism may depend on the reactivity of the starting materials and the reaction conditions, and side reactions, such as elimination or disproportionation, may also occur.
Scope and Limitations of the Fittig Reaction
The Fittig reaction is typically used for the synthesis of symmetric and unsymmetric biaryls and bivinyls, which have applications in materials science, medicinal chemistry, and natural product synthesis. The reaction is limited by the availability and reactivity of the starting materials, and the need for careful control of the reaction conditions to prevent side reactions and decomposition.
The Fittig reaction is particularly useful for the synthesis of biaryl compounds, which are important building blocks in natural product synthesis and materials science. For example, the Fittig reaction has been used in the synthesis of 1,1′-bi-2-naphthol (BINOL), a chiral molecule with numerous applications in organic synthesis and materials science. BINOL is often used as a chiral ligand in asymmetric catalysis, and its derivatives have been used in the synthesis of natural products, pharmaceuticals, and materials.
Applications of the Fittig Reaction
The Fittig reaction has numerous applications in organic synthesis and materials science. In addition to its use in the synthesis of biaryl compounds, the Fittig reaction can be used to synthesize a variety of bivinyl compounds with different substitution patterns and functional groups. The Fittig reaction can also be used in the synthesis of natural products and pharmaceuticals, where biaryl and bivinyl compounds are important building blocks.
Conclusion
In conclusion, the Fittig reaction is a useful tool in the synthetic chemist’s toolbox for the synthesis of biaryl and bivinyl compounds with different substitution patterns and functional groups. The reaction is typically carried out in the presence of metallic sodium or potassium in an inert solvent, and the reaction conditions are carefully controlled to optimize the yield and selectivity of the reaction. The Fittig reaction has numerous applications in organic synthesis and materials science, and its versatility and utility make it an important reaction in modern organic chemistry.
