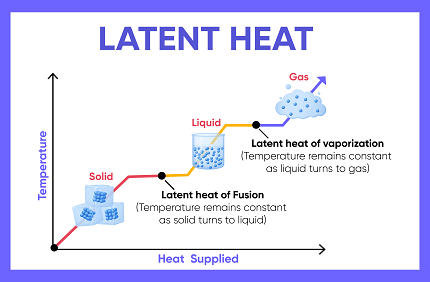
Latent heat refers to the amount of energy that is absorbed or released by a substance during a change in its state or phase, such as melting, boiling, or condensation. This energy is used to either break or form the intermolecular bonds between the molecules of the substance, without causing a temperature change.
The amount of energy required or released is specific to each substance and depends on the amount of material involved in the phase change. The heat absorbed or released during a phase change is known as latent heat because it does not result in a temperature change, but rather a change in the physical state of the substance.
For example, when ice melts, it absorbs a certain amount of energy called the latent heat of fusion, which is used to break the bonds between the molecules of ice without causing a temperature change. Similarly, when water boils, it absorbs the latent heat of vaporization, which is used to break the bonds between the molecules of liquid water to form water vapor, again without causing a temperature change.
What is latent heat
Latent heat refers to the amount of energy that is absorbed or released by a substance during a change in its state or phase, such as melting, boiling, or condensation. This energy is used to either break or form the intermolecular bonds between the molecules of the substance, without causing a temperature change.
The amount of energy required or released is specific to each substance and depends on the amount of material involved in the phase change. The heat absorbed or released during a phase change is known as latent heat because it does not result in a temperature change, but rather a change in the physical state of the substance.
For example, when ice melts, it absorbs a certain amount of energy called the latent heat of fusion, which is used to break the bonds between the molecules of ice without causing a temperature change. Similarly, when water boils, it absorbs the latent heat of vaporization, which is used to break the bonds between the molecules of liquid water to form water vapor, again without causing a temperature change.
When is latent heat
Latent heat occurs when a substance undergoes a change in its state or phase, such as melting, freezing, evaporation, or condensation. During these phase transitions, the substance absorbs or releases energy without any corresponding change in its temperature.
For example, when ice is heated, its temperature remains constant at 0 degrees Celsius until all of the ice has melted. This is because the energy being added is being used to break the bonds between the molecules in the ice, rather than increasing the temperature of the ice. The energy required to melt the ice is known as the latent heat of fusion.
Similarly, when water is boiled, its temperature remains constant at 100 degrees Celsius until all of the water has boiled away. This is because the energy being added is being used to break the bonds between the molecules in the liquid water, rather than increasing the temperature of the water. The energy required to boil the water is known as the latent heat of vaporization.
Where is latent heat
Latent heat is a form of energy that is either absorbed or released during a phase change of a substance, such as melting, freezing, evaporation, or condensation. This energy is stored within the substance and is not apparent as a change in temperature.
During a phase change, the substance absorbs or releases latent heat as the intermolecular bonds between the particles of the substance are either broken or formed, resulting in a change in the arrangement of the particles. The energy required or released during the phase change is referred to as latent heat.
For example, when ice is heated, its temperature remains constant at 0 degrees Celsius until all of the ice has melted. This is because the energy being added is being used to break the intermolecular bonds between the particles in the ice, rather than increasing the temperature of the ice. The energy required to melt the ice is the latent heat of fusion, which is stored within the resulting liquid water.
Similarly, when water is boiled, its temperature remains constant at 100 degrees Celsius until all of the water has boiled away. This is because the energy being added is being used to break the intermolecular bonds between the particles in the liquid water, rather than increasing the temperature of the water. The energy required to boil the water is the latent heat of vaporization, which is stored within the resulting water vapor.
In summary, latent heat is stored within a substance during a phase change and is not apparent as a change in temperature.
How is latent heat
Latent heat is the energy required or released during a phase change of a substance, such as melting, freezing, evaporation, or condensation. The process of latent heat involves breaking or forming intermolecular bonds between the particles of the substance, without any corresponding change in temperature.
During a phase change, the energy being added or removed from the substance is used to either break or form these intermolecular bonds. This results in a change in the arrangement of the particles of the substance, such as changing from a solid to a liquid or a liquid to a gas.
When a substance absorbs latent heat during a phase change, the energy is stored within the substance as potential energy, and when the substance releases latent heat during a phase change, the potential energy is converted to kinetic energy, increasing the motion of the particles in the substance.
For example, when ice is heated and melts into liquid water, the energy being added is used to break the intermolecular bonds between the particles in the ice, resulting in the potential energy being stored within the liquid water as latent heat. When the liquid water is cooled and freezes back into ice, the potential energy stored as latent heat is released, resulting in the temperature of the ice decreasing as the kinetic energy of the particles decreases.
In summary, latent heat is the energy stored within a substance during a phase change, and it is released or absorbed depending on whether the substance is changing from a higher energy state to a lower energy state or vice versa.
Production of latent heat
Latent heat is produced or absorbed during a phase change of a substance, such as melting, freezing, evaporation, or condensation. The amount of latent heat produced or absorbed depends on the specific substance and the conditions under which the phase change occurs.
During a phase change, the intermolecular bonds between the particles of the substance are either broken or formed, resulting in a change in the arrangement of the particles. The energy required or released during the phase change is referred to as latent heat. This energy is either absorbed or released by the substance, and it is stored within the substance as potential energy.
For example, when water is heated, it eventually reaches its boiling point and begins to evaporate into water vapor. During this process, energy is absorbed by the water, which is used to break the intermolecular bonds between the water molecules, resulting in the water molecules becoming a gas. The energy required to evaporate the water is the latent heat of vaporization, and it is stored within the resulting water vapor as potential energy.
Similarly, when water vapor condenses back into liquid water, energy is released as the intermolecular bonds between the water molecules are re-formed. The energy released during the condensation process is the latent heat of condensation, and it is released by the water vapor as kinetic energy, resulting in an increase in the temperature of the surrounding environment.
In summary, latent heat is produced or absorbed during a phase change of a substance, and it is stored within the substance as potential energy. The amount of latent heat produced or absorbed depends on the substance and the conditions under which the phase change occurs.
Case Study on latent heat
Sure, here is a case study on latent heat:
Case Study: Melting of Ice
Latent heat is the energy required to change the state of a substance without changing its temperature. It is also known as the heat of transformation or phase change. A common example of latent heat is the melting of ice. Let’s consider the case of a block of ice at a temperature of -10°C being brought into a room with a temperature of 20°C.
When the ice is brought into the room, heat energy from the surroundings is transferred to the ice. This heat energy causes the ice to melt and turn into water. The temperature of the ice remains constant at 0°C during this process, even though heat is being transferred to it.
The heat energy required to melt the ice is known as the latent heat of fusion. For water, the latent heat of fusion is 334 kJ/kg. This means that it takes 334 kJ of energy to melt one kilogram of ice into water at 0°C.
In the case of our block of ice, let’s assume it has a mass of 1 kg. The energy required to melt the ice can be calculated using the formula:
Q = m × L
where Q is the energy required, m is the mass of the ice, and L is the latent heat of fusion. Substituting the values, we get:
Q = 1 kg × 334 kJ/kg Q = 334 kJ
So, it takes 334 kJ of energy to melt our block of ice into water at 0°C. This energy is absorbed from the surroundings as the ice melts.
After the ice has melted, the water will start to warm up as it absorbs more heat energy from the surroundings. This heat energy will cause the temperature of the water to increase until it reaches the temperature of the room.
In summary, the melting of ice is a great example of latent heat. It is the energy required to change the state of a substance without changing its temperature. The heat energy required to melt ice is known as the latent heat of fusion, and for water, it is 334 kJ/kg. When a block of ice is brought into a room, heat energy from the surroundings is transferred to the ice, causing it to melt and absorb the latent heat required for the phase change.
White paper on latent heat
Introduction:
Latent heat is a term used to describe the amount of heat energy required to change the state of a substance from one form to another, without changing its temperature. This is because, during a phase change, the heat energy is used to break or form the intermolecular bonds between the molecules of the substance, rather than increasing the kinetic energy of the molecules themselves. This means that the temperature of the substance remains constant during the phase change, until all of the substance has undergone the phase change.
Examples of Latent Heat:
One of the most common examples of latent heat is the melting of ice. When ice is heated, the heat energy is used to break the intermolecular bonds holding the water molecules together in a solid state. Once all the bonds are broken, the ice melts, and the water begins to absorb the heat energy, increasing its temperature.
Another example is the evaporation of water. When water is heated, the heat energy is used to break the intermolecular bonds holding the water molecules together in a liquid state. Once all the bonds are broken, the water begins to evaporate, and the vapor begins to absorb the heat energy, increasing its temperature.
Latent Heat in Industry:
The concept of latent heat is widely used in many industrial processes. For example, during the process of refrigeration, latent heat is used to cool down the refrigerant. The refrigerant absorbs heat energy from the surrounding environment, which causes it to evaporate, absorbing the latent heat required for the phase change. Once the refrigerant has evaporated, it is passed through a compressor where it is compressed to a high pressure, which causes it to release the absorbed heat energy and condense back into a liquid state. This cycle repeats, allowing the refrigerant to continuously absorb heat energy from the surrounding environment and cool it down.
Another example of the use of latent heat in industry is during the process of steam generation. In order to generate steam, water is heated to its boiling point, at which point it begins to evaporate, absorbing the latent heat required for the phase change. The steam is then used to power turbines, which generate electricity. This process is widely used in thermal power plants to generate electricity.
Conclusion:
Latent heat is an important concept in thermodynamics and is used in many industrial processes. It is the amount of heat energy required to change the state of a substance from one form to another, without changing its temperature. Examples of latent heat include the melting of ice and the evaporation of water. In industry, latent heat is used in processes such as refrigeration and steam generation. Understanding the concept of latent heat is important in the design and operation of many industrial processes.
