In thermal physics, an isothermal process is a thermodynamic process during which the temperature of a system remains constant. This means that the internal energy of the system remains constant as well.
In an isothermal process, any energy transferred to or from the system is in the form of work or heat. The work done on or by the system is related to the change in volume of the system. For example, if a gas is contained in a piston and the piston is slowly compressed while the temperature is kept constant, the gas will do work by pushing the piston back. The amount of work done by the gas is equal to the force exerted by the gas multiplied by the distance the piston moves.
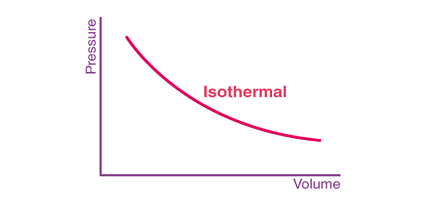
Isothermal processes are often studied in the context of ideal gases, which follow the ideal gas law: PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin. In an isothermal process for an ideal gas, the pressure and volume are inversely proportional to each other, and so the product of the pressure and volume remains constant.
What is Required Thermal Physics Isothermal
In thermal physics, to understand an isothermal process, we need to know the following:
- Temperature: The temperature of the system must be kept constant throughout the process. This can be achieved by placing the system in contact with a heat reservoir that has a large thermal capacity and can maintain a constant temperature.
- System: The system must be defined, and its properties, such as volume and pressure, must be measured or calculated.
- Work: The work done on or by the system must be measured or calculated. This involves determining the force applied to the system and the distance the system moves.
- Heat: The heat transferred to or from the system must be measured or calculated. This involves measuring the change in temperature of the heat reservoir that is in contact with the system.
- Ideal gas law: In the case of an ideal gas, we can use the ideal gas law (PV = nRT) to relate the pressure, volume, temperature, and number of moles of gas.
With these requirements, we can analyze an isothermal process and calculate the work done, heat transferred, and other properties of the system. This is important in many areas of science and engineering, such as thermodynamics, chemical engineering, and materials science.
When is Required Thermal Physics Isothermal
An isothermal process is required in thermal physics when we want to maintain a constant temperature of a system while changes occur within that system.
Isothermal processes are commonly used in many areas of science and engineering, including:
- Thermodynamics: Isothermal processes are used to study the behavior of systems that exchange heat with their surroundings. For example, an isothermal process can be used to calculate the work done by or on a gas during a reversible expansion or compression.
- Chemical engineering: Isothermal processes are used in the production and processing of chemicals and materials. For example, an isothermal reactor is used in many chemical reactions to maintain a constant temperature and control the rate of the reaction.
- Materials science: Isothermal processes are used to study the behavior of materials at different temperatures. For example, an isothermal process can be used to study the effect of temperature on the mechanical properties of a material.
Overall, isothermal processes are important in thermal physics as they allow us to maintain a constant temperature while studying the behavior of a system.
Where is Required Thermal Physics Isothermal
Isothermal processes are required in many areas of science and engineering where we need to maintain a constant temperature of a system while changes occur within that system. Some specific applications of isothermal processes are:
- Thermodynamics: Isothermal processes are fundamental to the study of thermodynamics. They are used to analyze the behavior of ideal gases and other systems that exchange heat with their surroundings.
- Chemistry: Isothermal processes are used in many chemical reactions and processes, such as catalysis and fermentation, to control the temperature and rate of the reaction.
- Materials science: Isothermal processes are used to study the behavior of materials at different temperatures. They are used to study the effect of temperature on the mechanical, electrical, and thermal properties of materials.
- Manufacturing: Isothermal processes are used in various manufacturing processes, such as the production of ceramics, metals, and polymers. Isothermal processes help to ensure the quality and consistency of the final product.
- Environmental science: Isothermal processes are used to study the behavior of the Earth’s atmosphere and climate. For example, the adiabatic lapse rate, which describes the decrease in temperature with altitude in the atmosphere, is an isothermal process.
Overall, isothermal processes are used in many areas of science and engineering where maintaining a constant temperature is important to study the behavior of a system or to control a process.
How is Required Thermal Physics Isothermal
An isothermal process in thermal physics involves maintaining a constant temperature of a system while changes occur within that system. There are different ways in which an isothermal process can be achieved, depending on the specific application.
One common method to achieve an isothermal process is by using a heat reservoir. A heat reservoir is a large system that has a very high thermal capacity and can maintain a constant temperature. The system that we want to study or control is placed in contact with the heat reservoir, and heat is transferred between the two systems until they reach thermal equilibrium, that is, until their temperatures are the same.
Another way to achieve an isothermal process is by using a thermostat. A thermostat is a device that can maintain a constant temperature in a system. It works by detecting the temperature of the system and then adjusting the heating or cooling to maintain the desired temperature.
In the case of ideal gases, an isothermal process can be achieved by placing the gas in contact with a heat reservoir and allowing it to expand or compress slowly while the temperature is kept constant. This can be done, for example, by placing the gas in a piston and allowing the piston to move slowly under a constant external pressure.
Overall, an isothermal process in thermal physics involves controlling the temperature of a system while changes occur within that system. This is achieved through the use of heat reservoirs, thermostats, and other techniques depending on the specific application.
Production of Thermal Physics Isothermal
The production of an isothermal process in thermal physics involves controlling the temperature of a system while changes occur within that system. There are several methods for producing an isothermal process, depending on the specific application.
One common method for producing an isothermal process is by using a heat reservoir. A heat reservoir is a large system that has a very high thermal capacity and can maintain a constant temperature. The system that we want to study or control is placed in contact with the heat reservoir, and heat is transferred between the two systems until they reach thermal equilibrium, that is, until their temperatures are the same.
Another method for producing an isothermal process is by using a thermostat. A thermostat is a device that can maintain a constant temperature in a system. It works by detecting the temperature of the system and then adjusting the heating or cooling to maintain the desired temperature.
In the case of ideal gases, an isothermal process can be produced by placing the gas in contact with a heat reservoir and allowing it to expand or compress slowly while the temperature is kept constant. This can be done, for example, by placing the gas in a piston and allowing the piston to move slowly under a constant external pressure.
The production of an isothermal process requires careful control of the temperature of the system. It is important to ensure that the temperature remains constant throughout the process, as any variation in temperature can affect the behavior of the system. The use of heat reservoirs, thermostats, and other techniques can help to achieve and maintain an isothermal process in a variety of applications.
Case Study on Thermal Physics Isothermal
One example of a case study on thermal physics isothermal is the ideal gas law. The ideal gas law describes the behavior of an ideal gas and is based on the assumption that the gas is composed of a large number of small particles that move randomly in all directions and collide with each other and with the walls of the container.
One of the important features of an ideal gas is that it obeys the equation of state PV=nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the gas constant, and T is the absolute temperature. This equation describes the relationship between the pressure, volume, and temperature of an ideal gas, and it can be used to analyze the behavior of gases under different conditions.
For an isothermal process, the temperature of the gas remains constant, and therefore the ideal gas law can be simplified to PV = constant. This means that if the volume of the gas increases, the pressure decreases, and if the volume decreases, the pressure increases, while the temperature remains constant.
An example of an isothermal process for an ideal gas is the reversible expansion or compression of the gas in a piston. In this process, the gas is contained in a cylinder with a movable piston. If the gas is expanded slowly and reversibly while maintaining a constant temperature, the pressure of the gas decreases and the volume of the gas increases. Similarly, if the gas is compressed slowly and reversibly while maintaining a constant temperature, the pressure of the gas increases and the volume of the gas decreases. This process can be analyzed using the ideal gas law and can be used to calculate the work done by or on the gas during the process.
Overall, the ideal gas law provides a useful tool for analyzing the behavior of gases under different conditions, including isothermal processes. The example of an isothermal reversible expansion or compression of an ideal gas in a piston demonstrates the application of the ideal gas law in studying the behavior of gases in practical applications.
White paper on Thermal Physics Isothermal
White Paper on Thermal Physics Isothermal: Overview, Applications, and Future Developments
Introduction
Thermal physics is a branch of physics that studies the behavior of matter and energy at a macroscopic level. One important aspect of thermal physics is the study of isothermal processes, which involve changes in a system while maintaining a constant temperature. This white paper provides an overview of thermal physics isothermal, its applications, and future developments.
Overview
An isothermal process is a process that occurs at constant temperature, which means that the internal energy of the system remains constant. In thermal physics, an isothermal process can be achieved by maintaining the system in contact with a heat reservoir or by using a thermostat. One of the important properties of an isothermal process is that the system is in thermal equilibrium with its surroundings, which means that the temperature of the system and its surroundings are the same.
One of the most important applications of thermal physics isothermal is the ideal gas law, which describes the behavior of an ideal gas under different conditions. The ideal gas law can be used to analyze the behavior of gases in isothermal processes, such as the reversible expansion or compression of a gas in a piston.
Applications
Thermal physics isothermal has a wide range of applications in various fields, including:
- Heat engines: The study of isothermal processes is important for the design and optimization of heat engines, which convert thermal energy into mechanical energy. For example, steam engines and gas turbines rely on isothermal processes to generate power.
- Climate modeling: The study of isothermal processes is important for understanding the behavior of the atmosphere and the Earth’s climate. Climate models use isothermal processes to simulate the exchange of heat and energy between the atmosphere and the Earth’s surface.
- Materials science: The study of isothermal processes is important for understanding the behavior of materials at different temperatures. For example, the properties of metals can change significantly as they are heated or cooled, and isothermal processes can be used to study these changes.
Future Developments
Thermal physics isothermal is an active area of research, and there are several areas where future developments are expected. Some of these areas include:
- Nanoscale thermal physics: The study of thermal physics at the nanoscale is an area of growing interest, as it has important applications in fields such as nanoelectronics and nanomaterials. Future developments in isothermal processes at the nanoscale are expected to have important implications for the design of new materials and devices.
- Energy storage: The study of isothermal processes is important for the development of new energy storage technologies, such as batteries and fuel cells. Future developments in isothermal processes in energy storage are expected to have important implications for the development of renewable energy technologies.
Conclusion
Thermal physics isothermal is an important branch of physics that has a wide range of applications in various fields. The study of isothermal processes is important for understanding the behavior of matter and energy at a macroscopic level, and it has important applications in fields such as heat engines, climate modeling, and materials science. Future developments in isothermal processes are expected to have important implications for the design of new materials and devices, as well as for the development of renewable energy technologies.
