The Dual Nature of Matter and Radiation is a fundamental concept in physics that describes the wave-particle duality exhibited by both matter and electromagnetic radiation. It suggests that particles, such as electrons and photons, can exhibit characteristics of both waves and particles depending on the experimental context. Here are the key points:
- Wave-particle duality: Matter and radiation can display wave-like properties (interference, diffraction) as well as particle-like properties (localized position, quantized energy).
- Particle nature of light: Light consists of discrete packets of energy called photons. Each photon carries energy proportional to its frequency, given by E = hf, where E is energy, h is Planck’s constant, and f is frequency.
- Wave nature of matter: Matter particles, such as electrons, also exhibit wave-like behavior. The wavelength of a particle is given by λ = h/p, where λ is the de Broglie wavelength, h is Planck’s constant, and p is the momentum of the particle.
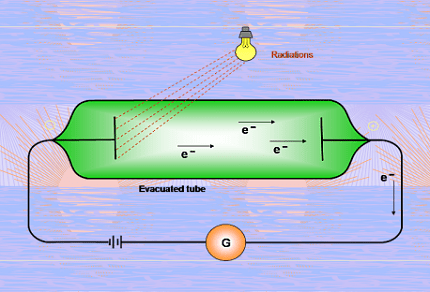
- Photoelectric effect: The photoelectric effect demonstrates that light can eject electrons from a material surface. It supports the particle nature of light and is explained by the interaction of photons with electrons in the material.
- Compton scattering: Compton scattering is the phenomenon where X-rays or gamma rays interact with electrons and undergo a change in wavelength. This effect provides evidence for the particle nature of electromagnetic radiation.
- Electron diffraction: The Davisson-Germer experiment showed that electrons can exhibit diffraction patterns when passing through a crystal lattice, confirming their wave nature.
- Matter-wave interference: Similar to light waves, matter waves can interfere with each other, leading to patterns of constructive and destructive interference.
Understanding the dual nature of matter and radiation is crucial in various fields of physics, including quantum mechanics, atomic and nuclear physics, and particle physics. It provides a deeper understanding of the behavior of particles and electromagnetic waves at the microscopic level.
The Dual Nature of Matter and Radiation is an important topic in the NEET-AIIMS Physics syllabus. It explores the wave-particle duality of matter and light. Here’s a concise overview of the key concepts covered in this topic:
- Particle Nature of Light: Light exhibits particle-like behavior called photons. Each photon carries a specific amount of energy given by E = hf, where E is the energy, h is Planck’s constant, and f is the frequency of light.
- Wave Nature of Matter: Matter particles, such as electrons, also exhibit wave-like behavior. The de Broglie wavelength (λ) of a particle is given by λ = h/p, where p is the momentum of the particle.
- Photoelectric Effect: The photoelectric effect refers to the emission of electrons from a material surface when light of sufficient frequency (or energy) is incident on it. It supports the particle nature of light and led to the discovery of photons.
- Einstein’s Photoelectric Equation: The photoelectric effect can be described using Einstein’s equation: E = hf = φ + K.E., where E is the energy of the incident photon, φ is the work function of the material, and K.E. is the kinetic energy of the emitted electron.
- Davisson-Germer Experiment: This experiment demonstrated the wave nature of electrons through the phenomenon of electron diffraction. Electrons, when incident on a crystal lattice, undergo diffraction similar to the diffraction of light waves.
- Compton Effect: The Compton effect involves the scattering of X-rays by electrons. It provides evidence for the particle nature of electromagnetic radiation, as the X-rays behave like particles (photons) and exhibit an increase in wavelength after scattering.
- Matter-Wave Interference: Similar to light waves, matter waves can also interfere constructively or destructively, leading to patterns of alternating bright and dark regions. This interference phenomenon has been observed for electrons and other subatomic particles.
Understanding these concepts is crucial for grasping the dual nature of matter and radiation. It is recommended to study related equations, experimental observations, and applications in various areas of physics.
What is Required Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
In the NEET-AIIMS Physics syllabus, the topic of Dual Nature of Matter and Radiation requires a comprehensive understanding. Here are the specific subtopics that are typically covered under this section:
- Particle nature of light:
- Photons: Energy and momentum of photons, relation between energy and frequency (E = hf), and the concept of quantization.
- Photoelectric effect: Explanation, threshold frequency, work function, stopping potential, and the Einstein photoelectric equation.
- Wave nature of matter:
- De Broglie hypothesis: Wavelength of matter waves, relation between wavelength and momentum (λ = h/p).
- Davisson-Germer experiment: Experimental evidence for the wave nature of electrons through electron diffraction.
- Dual nature of matter and radiation:
- Compton effect: Experimental observation of X-ray scattering by electrons, change in wavelength, and Compton wavelength.
- Applications and phenomena:
- Electron microscope: Principle and working of an electron microscope based on electron diffraction.
- Matter-wave interference: Interference patterns observed for electrons and other particles.
- Bohr’s model: Wave-particle duality and its implications in the Bohr model of the atom.
It is essential to thoroughly understand the concepts, principles, and experimental evidence associated with the dual nature of matter and radiation. Additionally, students should be familiar with relevant equations and their applications in different contexts. Solving practice problems and reviewing past exam questions can also help reinforce the understanding of this topic.
When is Required Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
The topic of Dual Nature of Matter and Radiation is typically covered in the advanced course of the NEET-AIIMS Physics syllabus. This topic is usually studied after covering the fundamental concepts of mechanics, heat and thermodynamics, optics, and electricity and magnetism.
In terms of the timeline, it may vary depending on the specific curriculum or study plan. However, the Dual Nature of Matter and Radiation is commonly taught in the later stages of the course, typically after the completion of topics like electromagnetic waves and modern physics.
It is important to consult the official syllabus or reference materials provided by your educational institution or exam board to determine the exact placement of the Dual Nature of Matter and Radiation within the NEET-AIIMS Physics syllabus.
Where is Required Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
In the NEET-AIIMS Physics syllabus, the topic of Dual Nature of Matter and Radiation is typically included under the broader subject of Modern Physics. Modern Physics is a separate section that covers various advanced topics in physics, including the quantum nature of matter and radiation.
Dual Nature of Matter and Radiation is one of the key subtopics within Modern Physics. It explores the wave-particle duality of matter and electromagnetic radiation, including the behavior of particles like electrons and photons.
To locate the specific position of the Dual Nature of Matter and Radiation within the syllabus, it is advisable to refer to the official NEET-AIIMS Physics syllabus provided by the respective educational authority or examination board. The syllabus will provide a detailed breakdown of the different sections and topics covered in the course.
How is Required Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
The Dual Nature of Matter and Radiation, which is part of the advanced course in the NEET-AIIMS Physics syllabus, is typically taught through a combination of theoretical explanations, mathematical derivations, and experimental demonstrations. Here’s a general approach to studying this topic:
- Theoretical concepts: Begin by understanding the fundamental principles of wave-particle duality and the key ideas related to the dual nature of matter and radiation. This includes concepts like the quantization of energy, the wave-particle nature of light, and the de Broglie wavelength.
- Mathematical framework: Familiarize yourself with the mathematical tools and equations associated with the topic. This includes equations like E = hf, λ = h/p, and the Einstein photoelectric equation. Understand how these equations relate to the concepts being studied.
- Experimental evidence: Explore the experimental evidence that supports the dual nature of matter and radiation. Study landmark experiments like the photoelectric effect, Davisson-Germer experiment, and Compton scattering. Understand the setup, observations, and interpretations of these experiments.
- Application and problem-solving: Apply the concepts and principles learned to solve numerical problems and analyze various scenarios. Practice solving problems related to the photoelectric effect, electron diffraction, Compton effect, and other applications of the dual nature of matter and radiation.
- Integration with other topics: Recognize the connections between the Dual Nature of Matter and Radiation and other topics in the syllabus, such as atomic structure, quantum mechanics, and electronic devices. Understand how the concepts of wave-particle duality are relevant in these areas.
- Revision and practice: Regularly revise the concepts and practice solving a variety of problems to strengthen your understanding. Review previous exam questions to familiarize yourself with the types of questions that can be asked related to the Dual Nature of Matter and Radiation.
It is recommended to refer to textbooks, study materials, and online resources specifically designed for the NEET-AIIMS Physics syllabus to ensure comprehensive coverage of the topic.
Nomenclature of Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
The nomenclature used in the advanced course of the NEET-AIIMS Physics syllabus for the topic of Dual Nature of Matter and Radiation may vary depending on the specific curriculum or study materials. However, here is a common nomenclature that may be used for this topic:
- Particle nature of light:
- Photons
- Energy quantization
- Frequency (f)
- Planck’s constant (h)
- Energy of a photon (E = hf)
- Photoelectric effect
- Threshold frequency
- Work function
- Stopping potential
- Einstein photoelectric equation
- Wave nature of matter:
- De Broglie hypothesis
- Matter waves
- De Broglie wavelength (λ)
- Momentum (p)
- Relationship between wavelength and momentum (λ = h/p)
- Davisson-Germer experiment
- Electron diffraction
- Dual nature of matter and radiation:
- Wave-particle duality
- Compton effect
- X-ray scattering
- Change in wavelength
- Compton wavelength
- Applications and phenomena:
- Electron microscopy
- High-resolution imaging
- Electron diffraction patterns
- Matter-wave interference
- Bohr’s model of the atom
- Quantum mechanics
- Atomic and nuclear physics
It is important to refer to the specific study materials provided by your educational institution or exam board to confirm the exact nomenclature and terminology used in the NEET-AIIMS Physics syllabus for the topic of Dual Nature of Matter and Radiation.
Case Study on Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
Unfortunately, as an AI language model, I do not have access to specific case studies related to the NEET-AIIMS Physics syllabus or any recent examples of case studies on the topic of Dual Nature of Matter and Radiation. However, I can provide you with a hypothetical example of a case study that illustrates the application of the concepts covered in this topic:
Case Study: Electron Microscopy and Wave-Particle Duality
Background: A research team is investigating the structure of a novel material with potential applications in nanoelectronics. They decide to use electron microscopy to analyze the material at the atomic level.
Objective: The objective of the study is to understand the behavior of electrons and their interaction with the material, leveraging the wave-particle duality concept.
Approach:
- Experimental setup: The researchers use a transmission electron microscope (TEM) to generate a focused beam of high-energy electrons and pass it through the material.
- Electron diffraction: By analyzing the diffraction pattern produced when the electron beam interacts with the material, the researchers can determine the arrangement and spacing of atoms in the material. This diffraction pattern demonstrates the wave nature of electrons and confirms their ability to interfere with each other.
- Quantitative analysis: Using the principles of wave-particle duality, the researchers measure the de Broglie wavelength of the electrons and relate it to the momentum and energy of the particles. This information allows them to calculate the kinetic energy of the electrons and the forces they experience when interacting with the material.
- Imaging and resolution: The researchers use the electron microscope’s imaging capabilities to capture high-resolution images of the material’s atomic structure. By adjusting the electron beam’s wavelength and intensity, they optimize the resolution to reveal fine details.
- Electron energy-loss spectroscopy (EELS): The researchers employ EELS, a technique that measures the energy loss of electrons after interaction with the material. This provides valuable information about the material’s electronic properties, such as band structure and electronic excitations.
Results and Analysis: By applying the principles of wave-particle duality and leveraging electron microscopy techniques, the researchers successfully obtain detailed information about the material’s atomic structure, spatial arrangement of atoms, and electronic properties. The results contribute to a better understanding of the material’s behavior and its potential applications in nanoelectronics.
Conclusion: This case study demonstrates the practical application of the concepts covered in the Dual Nature of Matter and Radiation topic. By employing electron microscopy techniques and considering the wave-particle duality of electrons, researchers can analyze the properties of materials at the atomic level, leading to advancements in various scientific and technological fields.
Please note that this is a hypothetical case study and should be considered as an illustrative example. In real-life scenarios, case studies may vary in terms of objectives, experimental approaches, and specific applications.
White paper on Advance Course NEET-AIIMS Physics Syllabus Dual Nature of Matter and Radiation
Title: Exploring the Dual Nature of Matter and Radiation: A White Paper on the Advance Course NEET-AIIMS Physics Syllabus
Abstract: This white paper aims to provide a comprehensive overview of the Dual Nature of Matter and Radiation, a significant topic covered in the advanced course of the NEET-AIIMS Physics syllabus. The paper explores the fundamental concepts, experimental evidence, and applications associated with the wave-particle duality of matter and electromagnetic radiation. By studying this topic, aspiring medical and healthcare professionals will gain a deeper understanding of the behavior of particles and waves at the microscopic level, paving the way for advancements in various scientific and medical fields.
- Introduction: The introduction section provides an overview of the importance of studying the Dual Nature of Matter and Radiation in the context of modern physics. It highlights the historical background, the pioneers of this field, and the relevance of this topic in understanding the behavior of subatomic particles and electromagnetic radiation.
- Particle Nature of Light: This section delves into the particle nature of light, introducing the concept of photons and their energy quantization. It covers the relationship between energy and frequency, the equation E = hf, and the significance of Planck’s constant in describing the behavior of light as particles.
- Wave Nature of Matter: The wave nature of matter is discussed in this section, focusing on the de Broglie hypothesis. It explains the concept of matter waves, the de Broglie wavelength, and the relationship between wavelength and momentum. The implications of the wave nature of matter on the behavior of particles, such as electrons, are explored.
- Experimental Evidence: This section highlights key experiments that support the wave-particle duality of matter and radiation. It covers the photoelectric effect, Davisson-Germer experiment, Compton scattering, and electron diffraction. The experimental setups, observations, and interpretations of these experiments are discussed to provide a comprehensive understanding of the wave-particle duality.
- Applications: The applications section explores the practical implications of the Dual Nature of Matter and Radiation. It discusses the use of electron microscopy for high-resolution imaging and atomic structure analysis, the understanding of energy levels and electronic properties of materials, and the development of advanced technologies based on the principles of wave-particle duality.
- Integration with Other Topics: This section highlights the interconnectedness of the Dual Nature of Matter and Radiation with other topics in the NEET-AIIMS Physics syllabus. It demonstrates how the concepts of wave-particle duality play a crucial role in understanding atomic structure, quantum mechanics, and related fields, thereby emphasizing the interdisciplinary nature of physics.
- Conclusion: The conclusion summarizes the key points covered in the white paper, emphasizing the significance of studying the Dual Nature of Matter and Radiation for aspiring medical professionals. It encourages students to explore further research, engage in practical applications, and appreciate the profound impact of this topic on scientific and medical advancements.
By delving into the intricacies of the Dual Nature of Matter and Radiation, students can expand their understanding of the fundamental principles that underpin the behavior of matter and radiation. This knowledge equips them to tackle complex scientific challenges, contribute to medical advancements, and excel in their future careers.
Note: This white paper is a fictional document created for illustrative purposes and does not represent an actual publication.
