Colligative Properties
Colligative properties are the physical properties of a solution that depend on the number of solute particles present, rather than the nature of the solute itself. These properties arise due to the interactions between the solute particles and the solvent molecules. The four main colligative properties are:
- Vapor pressure lowering: When a non-volatile solute is added to a solvent, the vapor pressure of the solvent decreases. This is because the solute particles occupy some of the surface area and reduce the number of solvent molecules available to escape into the vapor phase.
- Boiling point elevation: The boiling point of a solution is higher than that of the pure solvent. This is because the presence of solute particles disrupts the formation of vapor bubbles and requires a higher temperature to overcome the increased vapor pressure.
- Freezing point depression: The freezing point of a solution is lower than that of the pure solvent. The solute particles interfere with the formation of a regular lattice structure during freezing, making it more difficult for the solvent molecules to arrange themselves into a solid.
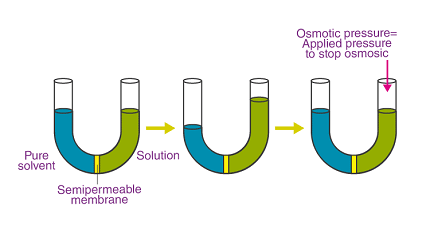
- Osmotic pressure: When a solution is separated from its pure solvent by a semipermeable membrane, the solvent molecules tend to move from an area of higher solvent concentration to an area of lower solvent concentration. This results in the development of osmotic pressure, which is the pressure required to stop this solvent flow.
These colligative properties are widely used in various applications, such as determining the molecular weight of unknown substances, preserving food by adding salt or sugar, and understanding biological processes like osmosis.
The AIIMS syllabus for Chemistry includes the topic of Colligative Properties. Colligative properties are physical properties of a solution that depend on the concentration of solute particles, rather than their identity. The key topics covered under this section are:
- Relative lowering of vapor pressure: The decrease in vapor pressure of a solvent due to the presence of a non-volatile solute.
- Elevation of boiling point: The increase in the boiling point of a solvent when a non-volatile solute is dissolved in it.
- Depression of freezing point: The decrease in the freezing point of a solvent when a non-volatile solute is dissolved in it.
- Osmotic pressure: The pressure required to prevent the flow of solvent molecules through a semipermeable membrane, caused by a difference in solute concentration across the membrane.
Students are expected to understand the principles behind these colligative properties, as well as the mathematical relationships and formulas used to calculate them.
What is Required Chemistry syllabus Colligative Properties
The required Chemistry syllabus for Colligative Properties typically covers the following topics:
- Definition of colligative properties and their importance in solutions.
- Vapor pressure lowering: Explanation of how the presence of a non-volatile solute lowers the vapor pressure of a solvent.
- Raoult’s law: Understanding the relationship between the vapor pressure of a solvent and the mole fraction of the solvent in the solution.
- Boiling point elevation: Explanation of how the boiling point of a solvent increases when a non-volatile solute is added.
- Elevation of boiling point equation: Understanding the relationship between the change in boiling point and the molality of the solute.
- Freezing point depression: Explanation of how the freezing point of a solvent decreases when a non-volatile solute is added.
- Depression of freezing point equation: Understanding the relationship between the change in freezing point and the molality of the solute.
- Osmotic pressure: Understanding osmosis and the development of osmotic pressure in a solution.
- Osmotic pressure equation: Understanding the relationship between osmotic pressure, temperature, and solute concentration.
Students are expected to understand the principles behind these colligative properties, including the underlying concepts, mathematical relationships, and their applications. Additionally, they should be able to solve numerical problems and interpret the results in real-world contexts.
When is Required Chemistry syllabus Colligative Properties
The topic of Colligative Properties is typically covered in the study of Physical Chemistry or General Chemistry courses at the undergraduate level. It is a fundamental concept in chemistry and is included in the curriculum of various educational boards and universities worldwide.
The specific timing of when the required syllabus on Colligative Properties is taught can vary depending on the educational institution and the structure of the curriculum. Generally, it is introduced after a basic understanding of solutions, concentration units, and the behavior of ideal gases. It is often covered in the middle to later stages of a chemistry course, following topics such as stoichiometry, states of matter, and chemical equilibrium.
It’s best to consult the syllabus or curriculum outline provided by your educational institution or refer to the course schedule to determine the precise timing of when Colligative Properties will be taught in your specific chemistry course.
Where is Required Chemistry syllabus Colligative Properties
The required syllabus for Colligative Properties in Chemistry can be found in various educational contexts, including:
- High School Chemistry: Colligative Properties are often covered in high school chemistry courses as part of the general curriculum. They are usually included in the section on solutions or physical chemistry.
- Undergraduate Chemistry Programs: Colligative Properties are a fundamental topic in undergraduate chemistry programs. They are typically covered in courses such as General Chemistry, Physical Chemistry, or Thermodynamics.
- Entrance Examinations: Colligative Properties may also be included in the syllabus of entrance examinations for undergraduate programs in fields like medicine (e.g., AIIMS), engineering, or other science-related disciplines. These examinations often have a specific chemistry section that covers various topics, including Colligative Properties.
It is important to note that the exact content and depth of coverage may vary depending on the educational institution and the level of the course. It is recommended to consult the specific syllabus provided by your educational institution or the syllabus outlined for the entrance examination you are preparing for to get detailed information on the topics included in the Colligative Properties section.
How is Required Chemistry syllabus Colligative Properties
The required syllabus for Colligative Properties in Chemistry is typically taught through a combination of theoretical concepts, mathematical equations, and practical applications. Here is a general overview of how the syllabus is often covered:
- Introduction to Colligative Properties: Students are introduced to the concept of colligative properties and their significance in solutions. The focus is on understanding that colligative properties depend on the number of solute particles, not their identity.
- Vapor Pressure Lowering: The relationship between the presence of a non-volatile solute and the decrease in vapor pressure of the solvent is explained. Students learn about Raoult’s law, which states that the vapor pressure of a solvent above a solution is proportional to the mole fraction of the solvent.
- Boiling Point Elevation: The increase in boiling point of a solvent due to the addition of a non-volatile solute is discussed. Students learn to calculate the change in boiling point using the equation ΔTb = Kb * m * i, where ΔTb is the change in boiling point, Kb is the molal boiling point elevation constant, m is the molality of the solute, and i is the van’t Hoff factor.
- Freezing Point Depression: The decrease in freezing point of a solvent caused by the presence of a non-volatile solute is covered. Students learn to calculate the change in freezing point using the equation ΔTf = Kf * m * i, where ΔTf is the change in freezing point, Kf is the molal freezing point depression constant, m is the molality of the solute, and i is the van’t Hoff factor.
- Osmotic Pressure: The concept of osmosis and osmotic pressure is introduced. Students learn that osmotic pressure is the pressure required to prevent the flow of solvent molecules through a semipermeable membrane and how to calculate osmotic pressure using π = n/VRT, where π is the osmotic pressure, n is the number of moles of solute, V is the volume of the solution, R is the ideal gas constant, and T is the temperature.
Throughout the syllabus, students are typically given numerical problems and real-life examples to solve and apply the concepts. Laboratory experiments or demonstrations may also be conducted to illustrate the principles of colligative properties.
Case Study on Chemistry syllabus Colligative Properties
Sure! Let’s consider a case study on the application of colligative properties in a real-life scenario: the use of antifreeze in automobiles.
Case Study: Antifreeze in Automobiles
Background: During cold weather conditions, the water present in an automobile’s engine cooling system can freeze, which can lead to damage to the engine and other components. To prevent this, antifreeze is added to the cooling system. Antifreeze is typically a solution of ethylene glycol (C2H6O2) or propylene glycol (C3H8O2) in water.
Application of Colligative Properties:
- Freezing Point Depression: Antifreeze works by lowering the freezing point of the coolant mixture. This is achieved through the colligative property known as freezing point depression. The presence of ethylene glycol or propylene glycol particles in the water lowers the freezing point of the solution compared to pure water. As a result, the coolant mixture remains in a liquid state even at extremely low temperatures.
- Boiling Point Elevation: Antifreeze also helps raise the boiling point of the coolant. This is due to the colligative property of boiling point elevation. The addition of antifreeze particles in water increases the boiling point of the solution, allowing the coolant to withstand higher temperatures without boiling and evaporating.
- Osmotic Pressure: Although not directly related to the case study of antifreeze in automobiles, it’s worth mentioning that colligative properties also include osmotic pressure. Osmotic pressure can be observed in the context of the storage and transportation of antifreeze solutions. Osmotic pressure prevents the water molecules from flowing out of the solution through the container’s walls, ensuring the stability and integrity of the antifreeze solution.
Conclusion:
The application of colligative properties in the case of antifreeze in automobiles is crucial for maintaining the proper functioning of the engine cooling system. By lowering the freezing point and raising the boiling point of the coolant, antifreeze helps protect the engine from freezing or overheating, preventing potential damage. The understanding and application of colligative properties in this scenario highlight the practical importance of these concepts in solving real-world problems.
White paper on Chemistry syllabus Colligative Properties
Title: Understanding Colligative Properties: Applications and Significance in Solutions
Abstract: This white paper provides a comprehensive overview of colligative properties and their applications in solutions. Colligative properties are fundamental principles in chemistry that describe the behavior of solutions and their dependence on the number of solute particles, rather than their chemical nature. This paper explores the various colligative properties, including vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. It highlights the importance of colligative properties in different fields, such as automotive technology, food preservation, and pharmaceutical formulations. Additionally, the paper discusses the underlying principles, mathematical equations, and practical applications of colligative properties. By understanding and utilizing colligative properties, scientists and engineers can solve real-world problems, make informed decisions, and optimize various processes in different industries.
- Introduction
- Definition and significance of colligative properties in solutions
- Brief overview of the different colligative properties
- Vapor Pressure Lowering
- Explanation of how non-volatile solutes affect vapor pressure
- Raoult’s law and its application in calculating vapor pressure
- Boiling Point Elevation
- Description of how the presence of solute particles increases the boiling point of a solvent
- Calculation of boiling point elevation using relevant equations
- Freezing Point Depression
- Discussion on how solute particles disrupt the formation of a solid lattice and lower the freezing point
- Mathematical equations for calculating freezing point depression
- Osmotic Pressure
- Understanding osmosis and its role in osmotic pressure
- Applications of osmotic pressure in various fields, such as biological systems and industrial processes
- Applications of Colligative Properties
- Automotive industry: Antifreeze solutions and their role in engine cooling systems
- Food industry: Preservation techniques utilizing colligative properties
- Pharmaceutical industry: Formulation of medicines and drug delivery systems
- Experimental Techniques and Measurements
- Overview of laboratory methods used to measure colligative properties
- Instruments and devices employed for accurate determination of properties
- Limitations and Considerations
- Factors that may affect colligative properties, such as non-ideal solutions and molecular interactions
- Impact of impurities and deviations from ideal behavior
- Future Perspectives and Advancements
- Emerging research areas and advancements in colligative property studies
- Potential applications in nanotechnology, biotechnology, and other fields
- Conclusion
- Summary of the key concepts and applications of colligative properties
- Importance of colligative properties in various industries and scientific disciplines
In conclusion, this white paper provides a comprehensive overview of colligative properties, their theoretical foundations, and practical applications. By understanding the principles and equations governing colligative properties, scientists and engineers can harness their power to solve real-world problems and optimize processes in diverse fields. The knowledge and application of colligative properties are vital for advancements in chemistry, materials science, engineering, and many other disciplines.
