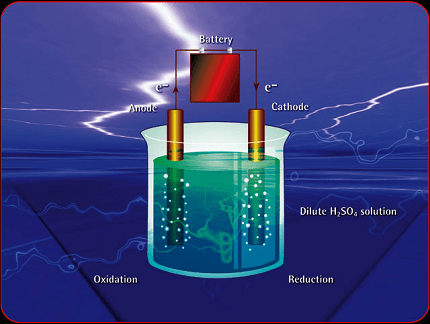
Laws of electrolysis
The AIIMS (All India Institute of Medical Sciences) entrance exam is a prestigious medical entrance examination in India. While I can provide you with information on the topic of “Laws of Electrolysis” in the Chemistry syllabus, it’s important to note that the syllabus for the AIIMS entrance exam may vary from year to year. Therefore, it’s always a good idea to consult the official AIIMS website or the exam notification for the most up-to-date information on the syllabus.
That being said, the topic of “Laws of Electrolysis” is a fundamental concept in electrochemistry. It primarily involves two laws known as Faraday’s laws of electrolysis. Here’s a brief overview:
- Faraday’s First Law of Electrolysis: This law states that the amount of any substance deposited or liberated at any electrode during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte. Mathematically, it can be expressed as: m=Z × I × t Where:
- m is the mass of the substance deposited or liberated
- Z is the electrochemical equivalent of the substance (a constant specific to the substance)
- I is the electric current passing through the electrolyte
- t is the time of electrolysis
- Faraday’s Second Law of Electrolysis: This law states that when the same quantity of electricity is passed through different electrolytes, the amounts of substances deposited or liberated at the respective electrodes are directly proportional to their equivalent weights. Mathematically, it can be expressed as: m2m1=E2E1Where:
- m1 and m2 are the masses of the substances deposited or liberated at the electrodes
- E1 and E2 are the equivalent weights of the substances
These laws are essential in understanding the quantitative aspects of electrolysis, such as calculating the amount of substance deposited or the time required for a specific reaction.
Remember to consult the official AIIMS website or the exam notification for the most accurate and updated information regarding the Chemistry syllabus for the AIIMS entrance exam.
How is Required AIIMS-SYLLABUS Chemistry syllabus Laws of electrolysis
In the context of the AIIMS entrance exam, the chemistry syllabus typically covers various topics related to general and organic chemistry. The laws of electrolysis, including Faraday’s laws, are usually part of the electrochemistry section of the syllabus. Here are some of the key topics that are usually included:
- Basic concepts of electrochemistry
- Conductance and electrolyte solutions
- Faraday’s laws of electrolysis and their applications
- Electrochemical cells and their types (galvanic cells and electrolytic cells)
- Standard electrode potential and its significance
- Nernst equation and its applications
- Electrochemical series and its interpretation
- Corrosion and its prevention
- Redox reactions and balancing of redox equations
- Batteries and fuel cells
These are some of the major topics related to electrochemistry and the laws of electrolysis that are typically covered in the chemistry syllabus for the AIIMS entrance exam. However, it’s important to refer to the official AIIMS sources for the most accurate and updated information on the syllabus.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Laws of electrolysis
Electroplating Process
Scenario:
A jewelry manufacturer wants to electroplate a piece of jewelry with a layer of gold to enhance its appearance. They plan to use an electrolytic cell for the electroplating process. The manufacturer needs to determine the current required and the time needed to deposit a specific mass of gold on the jewelry piece.
Solution:
- Electrochemical Equations: First, the manufacturer needs to determine the electrochemical equation for the process. In this case, the electrode reactions during the electroplating of gold can be represented as follows:
At the cathode (jewelry piece): Au^3+ + 3e^- → Au (reduction)
At the anode (usually an inert material like platinum): 2H2O → O2 + 4H+ + 4e^- (oxidation)
- Faraday’s First Law of Electrolysis: To determine the current required and the time needed, we can use Faraday’s First Law of Electrolysis, which states that the mass of substance deposited or liberated is directly proportional to the quantity of electric charge passed through the electrolyte.
m=Z × I × t
Here, m represents the mass of gold to be deposited, Z is the electrochemical equivalent of gold, I is the electric current, and t is the time of electrolysis.
- Determining the Current: To determine the current required, the manufacturer needs to know the desired mass of gold to be deposited and the electrochemical equivalent of gold.
Let’s assume the desired mass of gold to be deposited is 2 grams. The electrochemical equivalent of gold can be found from experimental data, which is approximately 0.0012 g/C (grams per coulomb).
- Calculating the Time: Once the current is known, the time needed to deposit the desired mass of gold can be calculated using the equation:
t=Z×Im
Substituting the values, we can calculate the time required.
Let’s assume the current used is 1 A (ampere):
t=0.0012×12=1666.67 seconds
Therefore, approximately 1666.67 seconds or around 27.8 minutes would be required to deposit 2 grams of gold on the jewelry piece using a current of 1 A.
Conclusion:
This case study demonstrates how the laws of electrolysis, specifically Faraday’s First Law, can be applied to determine the current and time required for an electroplating process. By understanding the electrochemical equations and using the concept of electrochemical equivalents, manufacturers can optimize the electroplating process to achieve desired results efficiently.
Note:
The values used in this case study are for illustrative purposes only and may not represent real-world conditions. Actual values may vary based on experimental data and specific electrochemical systems.
White paper on AIIMS-SYLLABUS Chemistry syllabus Laws of electrolysis
Title:
Principles, Applications, and Implications
Abstract:
The laws of electrolysis, formulated by Michael Faraday in the 19th century, are fundamental principles governing the behavior and quantitative aspects of electrolytic processes. This white paper provides a comprehensive overview of the laws of electrolysis, their underlying principles, applications in various industries, and implications for electrochemical research. Understanding these laws is crucial for optimizing electrochemical processes, such as electroplating, metal extraction, and energy storage systems.
- Introduction:
- Overview of electrolysis and its significance in diverse fields
- Importance of understanding the laws of electrolysis
- Faraday’s First Law of Electrolysis:
- Explanation of Faraday’s First Law and its mathematical representation
- Relationship between the amount of substance deposited and the quantity of electric charge passed
- Practical examples illustrating the application of the First Law
- Faraday’s Second Law of Electrolysis:
- Elaboration on Faraday’s Second Law and its mathematical expression
- Proportional relationship between amounts of different substances liberated and their respective chemical equivalents
- Interpretation of chemical equivalents and their significance in electrolysis
- Implications and Applications:
- Electroplating: Utilizing Faraday’s laws to control the deposition of metals on various surfaces
- Metal Extraction: Applying electrolysis for the extraction and refining of metals
- Energy Storage: Exploring the role of electrolysis in rechargeable batteries and fuel cells
- Industrial Applications: Highlighting the relevance of electrolysis in industrial processes
- Experimental Methods and Techniques:
- Techniques for measuring current, charge, and time in electrolysis experiments
- Determining electrochemical equivalents through experimental data
- Factors influencing electrolysis efficiency and strategies for optimization
- Advancements and Challenges:
- Modern developments in electrolysis techniques and technologies
- Challenges faced in practical applications and ongoing research efforts
- Potential future directions for advancements in electrolysis
- Conclusion:
- Recapitulation of the laws of electrolysis and their significance
- Summary of key applications and implications
- Call for continued research and innovation in the field of electrolysis
References:
- List of authoritative sources, scientific papers, and research studies used in compiling this white paper
Note:
This white paper provides a general overview of the laws of electrolysis, and it’s important to consult relevant literature and scientific sources for more detailed and specific information on the topic.
