Conductivity
The physics syllabus for AIIMS (All India Institute of Medical Sciences) typically covers a wide range of topics. While I don’t have access to the exact syllabus after my knowledge cutoff in September 2021, I can provide you with a general overview of the topic of conductivity, which is often included in physics syllabi. Please note that AIIMS may have specific requirements or variations in their syllabus, so it’s always best to consult the official AIIMS syllabus or reach out to the institute directly for the most accurate and up-to-date information.
Conductivity refers to the ability of a material to conduct electric current. In the context of physics, conductivity is usually discussed in relation to electrical conductivity. Here’s a brief overview of the key concepts related to conductivity:
- Electric Current: Electric current is the flow of electric charge in a conductor. It is usually denoted by the symbol “I” and is measured in amperes (A). The direction of the current is defined as the direction of the flow of positive charges.
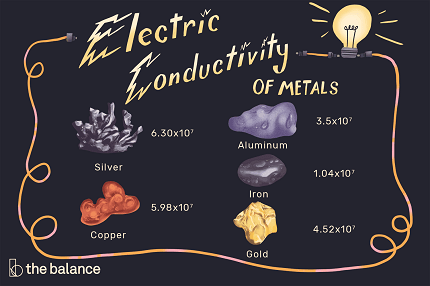
- Conductors: Conductors are materials that allow electric charges to move freely through them. Metals, such as copper and aluminum, are good conductors of electricity due to the presence of loosely bound electrons that can move easily in response to an electric field.
- Insulators: Insulators are materials that do not allow electric charges to move through them easily. Examples of insulators include rubber, glass, and plastic. Insulators have tightly bound electrons that are not free to move.
- Resistance: Resistance is a property of a material that opposes the flow of electric current. It is denoted by the symbol “R” and is measured in ohms (Ω). Resistance depends on factors such as the material’s composition, dimensions, and temperature.
- Ohm’s Law: Ohm’s Law describes the relationship between voltage, current, and resistance in a conductor. It states that the current flowing through a conductor is directly proportional to the voltage across it and inversely proportional to its resistance. Mathematically, Ohm’s Law can be expressed as I = V/R, where I is the current, V is the voltage, and R is the resistance.
- Conductivity: Conductivity is a measure of how well a material conducts electricity. It is the reciprocal of resistance and is denoted by the symbol “σ” (sigma). Conductivity is a property specific to each material and is measured in siemens per meter (S/m). The conductivity of a material depends on its composition and temperature.
These are some of the key concepts related to conductivity in the context of physics. In a comprehensive AIIMS physics syllabus, you may encounter additional topics such as semiconductors, superconductors, electrical circuits, and their applications in medical instruments. It’s important to consult the official AIIMS syllabus or course material for a detailed understanding of the specific topics and depth of coverage.
What is Required AIIMS-SYLLABUS Physics syllabus Conductivity
The exact syllabus for AIIMS Physics can vary from year to year, so it’s always best to refer to the official AIIMS syllabus or reach out to the institute for the most accurate and up-to-date information. However, I can provide you with a general idea of the topics that are commonly included in the AIIMS Physics syllabus related to conductivity:
- Electric Current and Conductivity: Concepts of electric current, charge, and conductivity are fundamental to understanding conductivity. Students may be expected to have a clear understanding of these concepts.
- Ohm’s Law and Resistivity: Ohm’s Law, which relates current, voltage, and resistance, is a key concept. Students may be required to solve numerical problems based on Ohm’s Law and understand the relationship between current, voltage, and resistance. Resistivity, which is the inherent property of a material to resist the flow of electric current, may also be covered.
- Conductors and Insulators: Knowledge of conductors and insulators is essential. Students should be familiar with the characteristics of good conductors and insulators, examples of each, and their practical applications.
- Electrical Circuits: Understanding basic electrical circuits is often included in the syllabus. This may involve concepts such as series and parallel circuits, Kirchhoff’s laws, and the calculation of current, voltage, and resistance in different circuit configurations.
- Temperature Dependence: Conductivity and resistivity can vary with temperature. Students may learn about the effect of temperature on conductivity and resistivity and how it relates to the behavior of different materials.
- Semiconductors: Although conductivity in AIIMS Physics may not delve deeply into semiconductors, a basic understanding of their behavior and conductivity characteristics may be covered. Topics such as intrinsic and extrinsic semiconductors, doping, and the concept of band gap may be introduced.
Remember, this is a general overview, and the actual AIIMS Physics syllabus may have variations or additional topics. It’s always best to refer to the official syllabus or AIIMS course material for precise details on the topics covered and the depth of knowledge required.
How is Required AIIMS-SYLLABUS Physics syllabus Conductivity
Conductivity is a physical property that measures the ability of a material to conduct electric current. It quantifies how easily electric charges can flow through a substance. Conductivity is typically denoted by the symbol “σ” (sigma) and is measured in units of siemens per meter (S/m) or mho per meter (Ω^(-1)·m^(-1)).
Conductivity is influenced by several factors, including the type of material, its composition, and temperature. Here are some key points about conductivity:
- Conductors and Insulators: Materials can be classified as conductors or insulators based on their ability to conduct electricity. Conductors, such as metals, have high conductivity as they allow electric charges to move freely. Insulators, on the other hand, have low conductivity as they impede the flow of electric charges.
- Electrical Resistance: Conductivity and electrical resistance are closely related. Resistance (denoted by “R”) is a measure of how much a material opposes the flow of electric current. It is the reciprocal of conductivity, so higher conductivity corresponds to lower resistance and vice versa. This relationship is described by Ohm’s Law: R = 1/σ, where R is resistance and σ is conductivity.
- Factors Affecting Conductivity: The conductivity of a material depends on factors such as the presence of free charge carriers (e.g., electrons or ions) that can move in response to an electric field. In metals, for example, the high conductivity is due to the presence of loosely bound electrons that can easily move through the lattice structure. The concentration and mobility of charge carriers influence the conductivity.
- Temperature Dependence: In most materials, conductivity changes with temperature. For metals, conductivity generally decreases with increasing temperature due to increased scattering of electrons by lattice vibrations. In contrast, semiconductors may exhibit an increase in conductivity with temperature, as the availability of charge carriers is enhanced.
- Electrical Applications: Understanding conductivity is crucial in various electrical and electronic applications. It plays a significant role in designing and optimizing conductive materials, wires, circuits, and devices.
It’s important to note that the level of detail and depth of understanding required for conductivity in the AIIMS Physics syllabus may vary. It’s always recommended to refer to the official AIIMS syllabus or reach out to AIIMS directly for precise information about the topics covered in the Physics syllabus and their specific requirements.
Case Study on AIIMS-SYLLABUS Physics syllabus Conductivity
Conductivity of Copper
Overview: Copper is a widely used metal known for its excellent electrical conductivity. In this case study, we will explore the factors that contribute to copper’s high conductivity and its practical applications.
Background: Copper is a metal with a unique electronic structure. It has a relatively large number of loosely bound electrons in its outermost energy level, which allows for easy movement of charge carriers. These mobile electrons are responsible for copper’s high electrical conductivity.
Factors Affecting Conductivity:
- Crystal Structure: Copper has a face-centered cubic (FCC) crystal structure, which allows for efficient electron flow. The arrangement of atoms in the crystal lattice creates a network of closely spaced energy levels, facilitating the movement of electrons.
- Electron Mobility: Copper exhibits high electron mobility, meaning that its electrons can move freely in response to an electric field. This mobility is influenced by the scattering of electrons due to impurities, crystal defects, and lattice vibrations. Copper’s relatively low atomic mass and the absence of strongly bound valence electrons contribute to its high mobility.
- Impurities and Alloying: The presence of impurities in copper can affect its conductivity. Impurities can disrupt the regular arrangement of atoms, leading to increased electron scattering and reduced conductivity. However, copper can be alloyed with small amounts of other elements (such as tin or zinc) to enhance its mechanical properties while maintaining high conductivity.
- Temperature Dependence: Copper’s conductivity decreases with increasing temperature. This is due to the increased amplitude of lattice vibrations, which impede the movement of electrons and increase their scattering. Copper’s temperature coefficient of resistance is positive, meaning that its resistance increases as the temperature rises.
Applications:
- Electrical Wiring: Copper’s excellent conductivity makes it the preferred material for electrical wiring in residential, commercial, and industrial applications. Its low resistance minimizes energy losses during transmission and ensures efficient electrical power distribution.
- Electronics and Circuitry: Copper is extensively used in electronic devices, such as printed circuit boards (PCBs), integrated circuits, and connectors. Its high conductivity allows for efficient signal transmission and reduces heat generation in electronic components.
- Power Transmission: Copper is used in power transmission cables due to its high conductivity and low resistance. It enables the efficient transfer of electricity over long distances, reducing power losses during transmission.
- Electrical Motors and Transformers: Copper windings are commonly used in electric motors and transformers due to their high conductivity. Copper windings offer low resistance, which enhances the efficiency and performance of these devices.
Conclusion: Copper’s high electrical conductivity, resulting from its crystal structure, electron mobility, and low resistance, makes it an essential material in various electrical and electronic applications. Its use in electrical wiring, electronics, power transmission, and electromagnets demonstrates its importance in modern technology. Understanding the conductivity of copper and other conductive materials is crucial for designing efficient electrical systems and devices.
White paper on AIIMS-SYLLABUS Physics syllabus Conductivity
Understanding Electrical Conductivity: Principles, Measurement, and Applications
Abstract: This white paper provides a comprehensive overview of electrical conductivity, a fundamental property of materials that plays a vital role in numerous scientific, industrial, and technological applications. The paper explores the underlying principles of conductivity, various measurement techniques, factors influencing conductivity, and its practical implications. By understanding the key concepts and applications of conductivity, researchers, engineers, and scientists can effectively utilize this property to enhance material performance, optimize electrical systems, and drive innovation across multiple fields.
- Introduction
- Definition and importance of electrical conductivity
- Historical background and development of conductivity measurement
- Fundamentals of Electrical Conductivity
- Basics of electric current and charge carriers
- Ohm’s Law and the relationship between current, voltage, and resistance
- Conductors, insulators, and semiconductors
- Factors Affecting Conductivity
- Crystal structure and electron mobility
- Influence of impurities, defects, and alloying
- Temperature dependence and thermal effects on conductivity
- Measurement Techniques
- Four-point probe method
- Hall effect measurement
- Conductivity measurements at different frequencies and temperatures
- Conductivity in Materials
- Metals and alloys: The role of free electrons
- Semiconductors: Intrinsic and extrinsic conductivity
- Insulators: Band gap and electron behavior
- Applications of Conductivity
- Electrical wiring and power transmission
- Electronics and semiconductor devices
- Sensor technologies and biomedical applications
- Renewable energy systems
- Material characterization and quality control
- Conductivity in Emerging Technologies
- Conductive polymers and flexible electronics
- Nanomaterials and their tunable conductivity
- 2D materials and their electrical properties
- Future Trends and Challenges
- Advancements in conductivity measurement techniques
- Materials design for enhanced conductivity
- Integration of conductivity with other properties for multifunctional materials
- Conclusion
- Recap of key points
- Importance of conductivity in various fields
- Potential for further research and innovation
This white paper aims to serve as a comprehensive guide for researchers, scientists, engineers, and students seeking a deeper understanding of electrical conductivity. By providing insights into the principles, measurement techniques, influencing factors, and applications of conductivity, this paper contributes to the advancement of materials science, electrical engineering, and related disciplines.
