Colligative properties
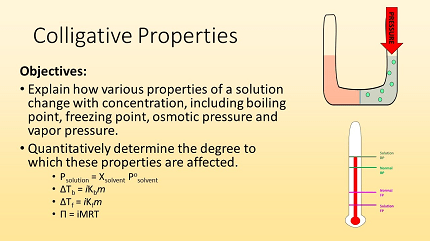
The AIIMS (All India Institute of Medical Sciences) syllabus for chemistry covers a wide range of topics, including colligative properties. Colligative properties are properties of solutions that depend on the concentration of solute particles, rather than the nature of the solute itself. The main colligative properties studied in this context are:
- Vapor Pressure Lowering: The vapor pressure of a solvent decreases when a non-volatile solute is added to it. This phenomenon is known as vapor pressure lowering. It can be described by Raoult’s law, which states that the vapor pressure of a solvent above a solution is directly proportional to the mole fraction of the solvent in the solution.
- Boiling Point Elevation: When a non-volatile solute is dissolved in a solvent, the boiling point of the solvent increases. The extent of boiling point elevation depends on the molality of the solute in the solution and the molal boiling point elevation constant of the solvent.
- Freezing Point Depression: Similar to boiling point elevation, the freezing point of a solvent decreases when a non-volatile solute is added. The magnitude of freezing point depression is proportional to the molality of the solute in the solution and the molal freezing point depression constant of the solvent.
- Osmotic Pressure: Osmotic pressure is the pressure required to stop the flow of solvent molecules across a semipermeable membrane due to the concentration difference between the solution and the pure solvent. It is directly proportional to the molarity of the solute and can be calculated using the formula π = n/VRT, where π is osmotic pressure, n is the number of moles of solute, V is the volume of the solution, R is the ideal gas constant, and T is the temperature in Kelvin.
The study of colligative properties is essential in various fields, including medicine and pharmacy, as it helps in understanding the behavior of solutions and their impact on biological systems.
What is Required AIIMS-SYLLABUS Chemistry syllabus Colligative properties
In the context of colligative properties, the following topics are usually covered in the AIIMS chemistry syllabus:
- Introduction to Solutions: Understanding the concept of solutions, solute, solvent, concentration, and types of solutions.
- Raoult’s Law: The application of Raoult’s law to explain vapor pressure lowering in solutions and the calculation of vapor pressure using Raoult’s law.
- Non-Volatile Solute: The effect of adding a non-volatile solute to a solvent on the vapor pressure of the solvent.
- Boiling Point Elevation: Understanding how the presence of a non-volatile solute affects the boiling point of a solvent and calculating the change in boiling point.
- Freezing Point Depression: Exploring the impact of a non-volatile solute on the freezing point of a solvent and calculating the change in freezing point.
- Osmotic Pressure: Understanding osmosis and osmotic pressure, calculating osmotic pressure using the formula π = n/VRT, and the application of osmotic pressure in biological systems.
- Colligative Properties and Biological Systems: Understanding the significance of colligative properties in biological systems such as cells and the impact of these properties on various physiological processes.
It’s important to note that the AIIMS syllabus may also include additional topics related to other areas of chemistry, so it’s recommended to refer to the official syllabus or the specific study material provided by AIIMS for a comprehensive understanding of the subject.
Remember to stay updated with the latest information and refer to official sources to ensure you are studying the most relevant topics as per the AIIMS syllabus.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Colligative properties
Case Study: Impact of Colligative Properties on Biological Systems
Introduction: Colligative properties play a crucial role in understanding the behavior of solutions, and their application is not limited to chemistry labs. In the field of medicine, colligative properties have significant implications for biological systems. This case study explores the impact of colligative properties on biological systems and highlights their relevance in medical science.
Background: Colligative properties are properties of solutions that depend on the concentration of solute particles rather than the nature of the solute itself. Four main colligative properties are widely studied: vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure. These properties have practical applications in various medical scenarios.
Case Study: A patient is admitted to the hospital with severe dehydration and electrolyte imbalance. The medical team immediately recognizes the importance of osmotic pressure in restoring the patient’s health.
- Osmotic Pressure and IV Fluids: The patient’s condition requires rehydration through intravenous (IV) fluids. The choice of IV fluids depends on their osmotic pressure. The medical team selects an appropriate IV fluid with an osmotic pressure matching that of the patient’s blood plasma to prevent further disturbances in the patient’s electrolyte balance.
- Osmosis and Cellular Function: Colligative properties, particularly osmosis, are critical for the normal functioning of cells. Understanding osmotic pressure is essential in maintaining cellular integrity. In the case of red blood cells (RBCs), the tonicity of the surrounding solution becomes crucial. An isotonic solution ensures that the osmotic pressure inside and outside the RBCs is balanced, preventing cell shrinkage or lysis.
- Cryopreservation Techniques: Cryopreservation, the preservation of biological materials at extremely low temperatures, utilizes colligative properties. Freezing point depression helps in preventing cellular damage during freezing and thawing processes. Cryoprotectants, such as glycerol or dimethyl sulfoxide (DMSO), are added to cell suspensions or tissues to lower their freezing points. This minimizes ice crystal formation, which can damage cellular structures.
- Pharmaceutical Formulations: Colligative properties also influence pharmaceutical formulations. For example, in controlled-release drug delivery systems, the selection of suitable solvents and excipients can be based on boiling point elevation and freezing point depression. These properties aid in controlling drug release rates and stability.
Conclusion: Colligative properties are not limited to laboratory experiments but have real-world applications in medical science. Understanding the principles of vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure is essential for healthcare professionals. The case study illustrates how colligative properties are employed to ensure proper fluid balance, cellular integrity, cryopreservation, and pharmaceutical formulations. Integrating this knowledge into medical practice helps in providing effective patient care and advancing the field of medicine.
White paper on AIIMS-SYLLABUS Chemistry syllabus Colligative properties
Title: Understanding Colligative Properties: A Comprehensive Analysis in the AIIMS Chemistry Syllabus
Abstract: This white paper aims to provide a detailed exploration of the colligative properties covered in the AIIMS (All India Institute of Medical Sciences) chemistry syllabus. Colligative properties are essential concepts in the study of solutions, with direct applications in the medical field. This paper delves into the key colligative properties, their underlying principles, and their significance in biological systems. By examining the AIIMS syllabus, this paper aims to equip students and healthcare professionals with a comprehensive understanding of colligative properties and their practical implications.
- Introduction
- Overview of the AIIMS chemistry syllabus
- Importance of colligative properties in the medical field
- Vapor Pressure Lowering
- Explanation of vapor pressure and its relation to solutions
- Raoult’s law and its application in predicting vapor pressure lowering
- Clinical relevance in understanding drug formulations and pharmacokinetics
- Boiling Point Elevation
- Definition of boiling point elevation and its connection to non-volatile solutes
- Calculation of boiling point elevation using molality and boiling point elevation constant
- Significance in intravenous fluid selection and controlled-release drug delivery systems
- Freezing Point Depression
- Explanation of freezing point depression caused by non-volatile solutes
- Determining the change in freezing point using molality and freezing point depression constant
- Importance in cryopreservation techniques and maintaining cellular integrity
- Osmotic Pressure
- Introduction to osmotic pressure and its role in osmosis
- Calculation of osmotic pressure using the ideal gas law equation
- Application in intravenous fluid therapy, red blood cell tonicity, and cell physiology
- Colligative Properties and Biological Systems
- Comprehensive examination of colligative properties’ impact on biological systems
- Case studies showcasing the practical relevance of colligative properties in medicine
- Discussion on the significance of colligative properties in various medical disciplines
- Conclusion
- Summary of the colligative properties covered in the AIIMS chemistry syllabus
- Emphasis on the importance of colligative properties in medical practice
- Recommendations for further study and practical applications
By comprehensively exploring the colligative properties within the AIIMS chemistry syllabus, this white paper provides a solid foundation for students and healthcare professionals seeking a deep understanding of these concepts. Understanding the principles and applications of vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure equips individuals with the knowledge necessary to address practical challenges in medical science and optimize patient care.
