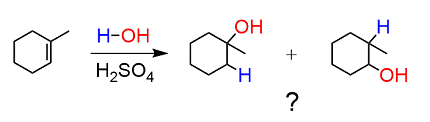
Acid-catalyzed hydration is a chemical reaction in which an acid catalyst (such as sulfuric acid or phosphoric acid) is used to add water (H2O) to an unsaturated compound, typically an alkene or alkyne. The reaction produces an alcohol as the final product.
The general equation for acid-catalyzed hydration of an alkene is:
RCH=CH2 + H2O → RCH2CH2OH
In this reaction, the alkene reacts with a water molecule in the presence of an acid catalyst to form a carbocation intermediate. The carbocation intermediate then reacts with a water molecule to form the final alcohol product.
Acid-catalyzed hydration is an important industrial process for the production of alcohols from unsaturated hydrocarbons. It is also commonly used in organic chemistry for the synthesis of various organic compounds.
What is Required Alkenes and Alkynes Acid catalysed hydration
In acid-catalyzed hydration, both alkenes and alkynes can undergo the reaction to form alcohols. However, the reaction conditions and mechanism may differ slightly between the two.
For alkenes, the reaction requires an unsaturated hydrocarbon containing a C=C double bond. The double bond is necessary for the reaction to occur as it provides the site for the addition of water. The alkene can be either symmetrical or unsymmetrical, meaning it can have identical or different substituents on each carbon atom of the double bond.
For alkynes, the reaction requires an unsaturated hydrocarbon containing a C≡C triple bond. The triple bond is necessary for the reaction to occur as it provides the site for the addition of two water molecules. As with alkenes, the alkyne can be either symmetrical or unsymmetrical.
In both cases, the reaction requires an acid catalyst such as sulfuric acid or phosphoric acid to facilitate the addition of water to the unsaturated hydrocarbon. The acid catalyst protonates the double or triple bond, making it more susceptible to nucleophilic attack by the water molecule. The resulting carbocation intermediate then undergoes a hydride shift to form a more stable carbocation, which then reacts with another water molecule to form the final alcohol product.
When is Required Alkenes and Alkynes Acid catalysed hydration
Acid-catalyzed hydration is typically used to convert alkenes and alkynes into alcohols. This reaction is an important tool for organic chemists as it allows them to synthesize a wide range of alcohol compounds from relatively simple starting materials.
Alkenes are commonly used in acid-catalyzed hydration reactions, particularly in the industrial production of ethanol from ethylene. This reaction is used to make ethanol for use as a fuel, as well as in the production of solvents and other chemicals.
Alkynes are less commonly used in acid-catalyzed hydration reactions, but they can also be converted to alcohols through this process. One example is the conversion of propyne to propanol using sulfuric acid as the catalyst.
Overall, acid-catalyzed hydration is a versatile reaction that can be used to convert a wide range of unsaturated hydrocarbons into alcohols. The reaction conditions and mechanism may vary depending on the specific starting material, but the basic principle of adding water to the unsaturated bond remains the same.
Where is Required Alkenes and Alkynes Acid catalysed hydration
Acid-catalyzed hydration of alkenes and alkynes can be carried out in a variety of settings, including industrial, academic, and laboratory environments.
In industrial settings, acid-catalyzed hydration is commonly used to produce alcohols from unsaturated hydrocarbons. One example is the production of ethanol from ethylene, which is used as a fuel and in the production of chemicals and solvents. Other examples include the production of tert-butyl alcohol from isobutene, and the production of n-butanol from 1-butene.
In academic and laboratory settings, acid-catalyzed hydration is used in the synthesis of a wide range of organic compounds. For example, it can be used to convert an alkene or alkyne precursor into an alcohol functional group, which can then be further manipulated to form more complex molecules. This reaction is often carried out using small-scale reactions in flasks or test tubes.
Overall, acid-catalyzed hydration of alkenes and alkynes can be carried out in a variety of settings depending on the specific application. The reaction is widely used in the production of chemicals and fuels, as well as in academic and laboratory settings for the synthesis of organic compounds.
How is Required Alkenes and Alkynes Acid catalysed hydration
Acid-catalyzed hydration of alkenes and alkynes is typically carried out by adding an acid catalyst to a solution of the unsaturated hydrocarbon and water. The acid catalyst protonates the double or triple bond, making it more susceptible to nucleophilic attack by the water molecule.
For alkenes, the reaction mechanism involves the following steps:
- Protonation: The acid catalyst, usually sulfuric acid or phosphoric acid, protonates the double bond in the alkene, forming a carbocation intermediate.
- Nucleophilic addition: Water acts as a nucleophile, attacking the carbocation intermediate and adding a hydroxyl group (-OH) to one of the carbon atoms of the double bond.
- Deprotonation: Another water molecule removes a proton from the carbon atom that did not receive the hydroxyl group, forming the final alcohol product.
The overall reaction can be represented as follows:
RCH=CH2 + H2O + H+ → RCH2CH2OH
For alkynes, the reaction mechanism involves the following steps:
- Protonation: The acid catalyst protonates the triple bond in the alkyne, forming a vinyl carbocation intermediate.
- Nucleophilic addition: Two molecules of water act as nucleophiles, attacking the carbocation intermediate and adding hydroxyl groups to both carbon atoms of the triple bond.
- Deprotonation: Protons are removed from the two carbon atoms, forming the final alcohol product.
The overall reaction can be represented as follows:
RC≡CH + 2H2O + 2H+ → RCH(OH)CH2OH
Overall, acid-catalyzed hydration of alkenes and alkynes involves the addition of water across the double or triple bond, resulting in the formation of an alcohol functional group. The specific reaction conditions and mechanism may vary depending on the starting material and reaction conditions used.
Production of Alkenes and Alkynes Acid catalysed hydration
The production of alkenes and alkynes by acid-catalyzed hydration is not a common process, as these unsaturated hydrocarbons are typically produced by other means, such as cracking of petroleum or natural gas.
However, in principle, alkenes and alkynes can be produced by acid-catalyzed dehydration of alcohols, which is the reverse of acid-catalyzed hydration. In this reaction, an alcohol molecule is heated in the presence of an acid catalyst, causing it to lose a molecule of water and form an alkene or alkyne.
For example, ethanol can be dehydrated using sulfuric acid as the catalyst to form ethylene, which is a common industrial feedstock for the production of a wide range of chemicals and plastics. The reaction can be represented as follows:
CH3CH2OH → CH2=CH2 + H2O
Similarly, 1-butanol can be dehydrated using sulfuric acid to form 1-butene, which can be further converted to other chemicals or fuels. The reaction can be represented as follows:
CH3CH2CH2CH2OH → CH3CH2CH=CH2 + H2O
Overall, while acid-catalyzed hydration is a common method for synthesizing alcohols from alkenes and alkynes, the reverse reaction of acid-catalyzed dehydration is not commonly used for the production of alkenes and alkynes. Other methods, such as cracking of petroleum or natural gas, are typically used for their production.
Case Study on Alkenes and Alkynes Acid catalysed hydration
One common industrial application of acid-catalyzed hydration is the production of ethanol from ethylene. Ethylene is a simple, unsaturated hydrocarbon that is readily available from petroleum refining and natural gas processing. Ethanol, on the other hand, is a valuable commodity chemical that is used as a solvent, fuel additive, and starting material for the production of other chemicals.
The reaction involves the addition of water to ethylene in the presence of an acid catalyst, typically phosphoric acid or sulfuric acid. The reaction proceeds via a carbocation intermediate, with the acid catalyst protonating the double bond and making it more susceptible to nucleophilic attack by the water molecule. The reaction can be represented as follows:
CH2=CH2 + H2O → CH3CH2OH
The reaction is typically carried out at high temperature and pressure to maximize yield and conversion. The resulting ethanol product is then purified by distillation and other techniques.
In addition to its use in the production of ethanol, acid-catalyzed hydration is also used in the synthesis of a wide range of organic compounds in academic and laboratory settings. For example, it can be used to convert an alkene or alkyne precursor into an alcohol functional group, which can then be further manipulated to form more complex molecules.
Overall, acid-catalyzed hydration is an important reaction in the production of chemicals and fuels, as well as in academic and laboratory settings for the synthesis of organic compounds.
White paper on Alkenes and Alkynes Acid catalysed hydration
Introduction:
Alkenes and alkynes are unsaturated hydrocarbons that contain carbon-carbon double and triple bonds, respectively. These types of hydrocarbons are widely used in the chemical industry as starting materials for the production of a wide range of chemicals and plastics. One common method for the synthesis of alcohols from alkenes and alkynes is acid-catalyzed hydration. This paper will provide an overview of acid-catalyzed hydration of alkenes and alkynes, including the reaction mechanism, reaction conditions, and applications.
Reaction Mechanism:
The acid-catalyzed hydration of alkenes and alkynes involves the addition of water across the double or triple bond, resulting in the formation of an alcohol functional group. The reaction proceeds via a carbocation intermediate, which is formed by the protonation of the double or triple bond by the acid catalyst. The water molecule then acts as a nucleophile, attacking the carbocation intermediate and adding a hydroxyl group (-OH) to one or both of the carbon atoms of the double or triple bond. Finally, another water molecule removes a proton from the carbon atom that did not receive the hydroxyl group, forming the final alcohol product.
Reaction Conditions:
The acid-catalyzed hydration reaction is typically carried out at high temperature and pressure to maximize yield and conversion. The acid catalyst used can vary depending on the starting material and reaction conditions. Commonly used acid catalysts include sulfuric acid, phosphoric acid, and hydrochloric acid. The molar ratio of water to starting material can also affect the reaction rate and yield. For alkenes, a 1:1 molar ratio of water to starting material is typically used, while for alkynes, a 2:1 molar ratio of water to starting material is necessary to add a hydroxyl group to both carbon atoms of the triple bond.
Applications:
Acid-catalyzed hydration of alkenes and alkynes has a wide range of applications in the chemical industry. One common application is the production of ethanol from ethylene, which is a valuable commodity chemical used as a solvent, fuel additive, and starting material for the production of other chemicals. Other applications include the synthesis of alcohols from alkenes and alkynes for use as solvents, intermediates, and starting materials for the production of other chemicals.
Conclusion:
Acid-catalyzed hydration is a widely used method for the synthesis of alcohols from alkenes and alkynes. The reaction proceeds via a carbocation intermediate and is typically carried out at high temperature and pressure in the presence of an acid catalyst. The reaction has a wide range of applications in the chemical industry, including the production of ethanol, solvents, intermediates, and starting materials for the production of other chemicals.