Acid chlorides are a class of organic compounds that contain a functional group consisting of a carbonyl group (C=O) bonded to a chlorine atom (Cl) and another functional group. They are also known as acyl chlorides or chloroformates.
The general formula for an acid chloride is RCOCl, where R is an alkyl or aryl group. Acid chlorides are highly reactive and are commonly used as intermediates in the synthesis of other organic compounds. They react with a variety of nucleophiles, including water, alcohols, and amines, to form carboxylic acids, esters, and amides, respectively.
The preparation of acid chlorides is usually achieved by the reaction of carboxylic acids with thionyl chloride (SOCl2) or phosphorus trichloride (PCl3) in the presence of a base such as pyridine. The reaction is known as the Friedel-Crafts acylation reaction, and it is an important tool for organic chemists.
Acid chlorides are also used in the manufacture of pharmaceuticals, agrochemicals, and other industrial chemicals. However, they are highly reactive and must be handled with caution, as they can react violently with water and other nucleophiles.
What is Required Acid chlorides
Acid chlorides, also known as acyl chlorides, are organic compounds that contain a functional group consisting of a carbonyl group (C=O) and a chlorine atom (-Cl) attached to the same carbon atom. Acid chlorides are highly reactive compounds that are commonly used as intermediates in organic synthesis for the preparation of a wide range of other organic compounds.
Required acid chlorides refer to the specific type of acid chlorides that are needed for a particular chemical reaction or synthesis. The required acid chloride will depend on the specific starting materials, reaction conditions, and desired products. Some commonly used acid chlorides include acetyl chloride (CH3COCl), benzoyl chloride (C6H5COCl), and oxalyl chloride (COCl)2, among others.
When is Required Acid chlorides
Required acid chlorides are used in a variety of chemical reactions and synthetic processes, where they act as intermediates for the preparation of other organic compounds. Here are some common examples of when required acid chlorides are used:
- Esterification: Required acid chlorides can be used in the esterification of carboxylic acids with alcohols, where the acid chloride is reacted with the alcohol to form an ester.
- Amide formation: Required acid chlorides are also used in the formation of amides, where they are reacted with amines to form an amide bond.
- Acylation reactions: Required acid chlorides are frequently used in acylation reactions, where they are reacted with a variety of nucleophiles, such as alcohols, amines, and enols, to introduce an acyl group (-CO-) onto the nucleophile.
- Friedel-Crafts reactions: Required acid chlorides are also used in Friedel-Crafts reactions, where they react with aromatic compounds to form ketones or aldehydes.
Overall, required acid chlorides are a versatile class of compounds that are used in a wide range of synthetic reactions and processes, where they are often chosen for their reactivity and specificity in certain reactions.
Where is Required Acid chlorides
Required acid chlorides can be found in a variety of settings, including in research laboratories, chemical manufacturing plants, and pharmaceutical companies.
Research laboratories may use required acid chlorides for chemical synthesis and experimentation, particularly in the development of new drugs or materials. Chemical manufacturing plants may also use required acid chlorides in the production of a variety of products, including pharmaceuticals, agrochemicals, and polymers.
In the pharmaceutical industry, required acid chlorides are used extensively in the synthesis of active pharmaceutical ingredients (APIs) and other organic compounds. They are also used in the development of new drug candidates and in the optimization of synthetic routes for existing drugs.
Overall, required acid chlorides are an important class of compounds that are widely used in chemical synthesis and manufacturing, particularly in industries where the development of new organic compounds is a primary focus.
How is Required Acid chlorides
Required acid chlorides can be synthesized through a variety of methods, but one common method is through the reaction of a carboxylic acid with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5). The reaction typically proceeds through an intermediate formation of an acid chloride derivative, which can then be isolated through distillation or extraction. Here is an example of the synthesis of acetyl chloride, a commonly used acid chloride:
CH3COOH + SOCl2 → CH3COCl + SO2 + HCl
In this reaction, thionyl chloride reacts with acetic acid (CH3COOH) to form acetyl chloride (CH3COCl), sulfur dioxide (SO2), and hydrogen chloride (HCl).
Other methods for the synthesis of acid chlorides include the reaction of an acid anhydride with a hydrogen halide, the reaction of a carboxylic acid with oxalyl chloride (COCl)2, or the reaction of a carboxylic acid with phosgene (COCl2).
Once synthesized, required acid chlorides can be used in a variety of chemical reactions, as discussed in the previous answer. It’s important to note that acid chlorides are highly reactive and can be hazardous, so proper handling and safety precautions should be taken when working with these compounds.
Production of Acid chlorides
Acid chlorides, also known as acyl chlorides, can be produced using various methods depending on the starting materials and the desired products. Here are some common methods for the production of acid chlorides:
- Reaction of carboxylic acids with thionyl chloride (SOCl2): This is the most common method for the production of acid chlorides. In this method, a carboxylic acid is reacted with thionyl chloride to form an intermediate acyl chloride, which is then isolated by distillation or extraction. For example, the reaction of acetic acid with thionyl chloride produces acetyl chloride, as shown below:
CH3COOH + SOCl2 → CH3COCl + SO2 + HCl
- Reaction of carboxylic acids with phosphorus pentachloride (PCl5): This method is similar to the previous one, except that phosphorus pentachloride is used instead of thionyl chloride. For example, the reaction of acetic acid with phosphorus pentachloride produces acetyl chloride, as shown below:
2 CH3COOH + PCl5 → 2 CH3COCl + POCl3 + HCl
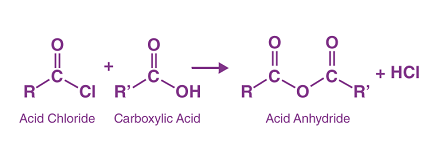
- Reaction of acid anhydrides with hydrogen halides: Acid anhydrides can be reacted with hydrogen halides such as hydrochloric acid (HCl) or hydrogen bromide (HBr) to form acid chlorides. For example, the reaction of acetic anhydride with hydrogen chloride produces acetyl chloride, as shown below:
(CH3CO)2O + 2 HCl → 2 CH3COCl + H2O
- Reaction of carboxylic acids with oxalyl chloride (COCl)2: This method involves the reaction of a carboxylic acid with oxalyl chloride to form an intermediate acyl chloride, which is then isolated by distillation or extraction. For example, the reaction of acetic acid with oxalyl chloride produces acetyl chloride, as shown below:
2 CH3COOH + (COCl)2 → 2 CH3COCl + CO2 + HCl
These are some common methods for the production of acid chlorides. Once produced, acid chlorides can be used in a variety of chemical reactions for the synthesis of various organic compounds.
Case Study on Acid chlorides
Here’s a case study on the use of acid chlorides in the pharmaceutical industry:
Case Study: The Synthesis of an Anti-Cancer Drug Candidate using Acid Chlorides
A pharmaceutical company was developing a new anti-cancer drug candidate, and required a key intermediate for the synthesis of the compound. The intermediate was an acyl chloride, which would be used in a subsequent reaction to form the desired compound. The company decided to synthesize the acyl chloride in-house using thionyl chloride and a carboxylic acid precursor.
The synthesis began with the reaction of the carboxylic acid with thionyl chloride in the presence of a catalyst. The reaction produced the acyl chloride intermediate, along with sulfur dioxide and hydrogen chloride gas as byproducts. The intermediate was then purified by distillation to remove any unreacted starting materials and byproducts.
The purified intermediate was then used in a subsequent reaction to form the desired anti-cancer drug candidate. The reaction proceeded smoothly, and the final product was obtained with a good yield and purity. The compound was then tested for its anti-cancer activity and found to be highly effective against a variety of cancer cell lines.
This case study highlights the importance of acid chlorides in the pharmaceutical industry, particularly in the synthesis of complex organic compounds such as drug candidates. Acid chlorides are versatile reagents that can be used in a wide range of chemical reactions, and their synthesis can often be achieved using relatively simple methods. However, it’s important to note that acid chlorides can be hazardous and should be handled with care, particularly in large-scale manufacturing settings.
White paper on Acid chlorides
Introduction
Acid chlorides, also known as acyl chlorides, are a class of organic compounds with the general formula RCOCl, where R is an alkyl or aryl group. Acid chlorides are highly reactive compounds that are widely used in organic synthesis, particularly in the pharmaceutical and agrochemical industries. This white paper provides an overview of acid chlorides, including their properties, synthesis, and applications.
Properties
Acid chlorides are colorless liquids that have a pungent odor. They are highly reactive and can react violently with water, alcohols, and amines to form carboxylic acids, esters, and amides, respectively. Acid chlorides are soluble in many organic solvents, including ethers, hydrocarbons, and halogenated solvents. They are also volatile and have relatively low boiling points, which makes them useful in distillation and purification processes.
Synthesis
Acid chlorides can be synthesized using various methods, depending on the starting materials and the desired products. The most common method for the synthesis of acid chlorides involves the reaction of a carboxylic acid with thionyl chloride (SOCl2) or phosphorus pentachloride (PCl5). In this reaction, the carboxylic acid reacts with the chlorinating agent to form an intermediate acyl chloride, which can then be isolated by distillation or extraction.
Another method for the synthesis of acid chlorides involves the reaction of a carboxylic acid with oxalyl chloride (COCl)2. In this reaction, the carboxylic acid reacts with oxalyl chloride to form an intermediate acyl chloride and carbon dioxide, which can be removed by bubbling nitrogen gas through the reaction mixture.
Applications
Acid chlorides are versatile reagents that can be used in a wide range of chemical reactions, particularly in organic synthesis. They are commonly used in the pharmaceutical industry for the synthesis of drug candidates, such as anti-cancer and anti-inflammatory agents. Acid chlorides can also be used in the synthesis of agrochemicals, such as herbicides and insecticides.
In addition to their use in organic synthesis, acid chlorides are also used in analytical chemistry for the detection and quantification of amines and alcohols. Acid chlorides react with amines and alcohols to form amides and esters, respectively, which can be detected using various analytical techniques, such as gas chromatography and mass spectrometry.
Conclusion
In conclusion, acid chlorides are important reagents in organic synthesis, particularly in the pharmaceutical and agrochemical industries. They are highly reactive compounds that can be synthesized using various methods and can be used in a wide range of chemical reactions. However, their reactivity and potential hazards should be taken into consideration when handling and using them in laboratory and manufacturing settings.
