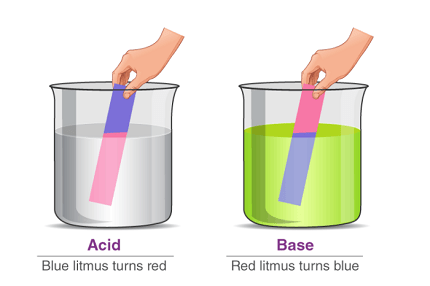
Acids are a class of chemical compounds that typically have a sour taste and can turn blue litmus paper red. They are also known to react with certain metals to produce hydrogen gas and with bases to form salts and water. Acids are characterized by the presence of hydrogen ions (H+) when dissolved in water, which gives them their acidic properties.
Acids can be classified as either strong or weak, depending on their ability to dissociate in water. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), dissociate completely in water to produce a high concentration of hydrogen ions. Weak acids, such as acetic acid (CH3COOH) and citric acid (C6H8O7), only partially dissociate in water, resulting in a lower concentration of hydrogen ions.
Some common uses of acids include:
- In the production of fertilizers, such as sulfuric acid used in the production of phosphate fertilizers
- In the production of synthetic fibers, such as nylon and polyester
- In the food industry as preservatives and flavor enhancers, such as citric acid in soft drinks
- In cleaning products, such as vinegar (which contains acetic acid) used to remove stains and odors from surfaces
- In medical treatments, such as the use of hydrochloric acid to regulate stomach acidity in people with certain digestive disorders.
It is important to handle acids with care, as they can be corrosive and cause chemical burns if not used properly.
What is Required Acids s-Block Elements
The term “Required Acids” is not a commonly used term in chemistry. However, I can provide some information about s-block elements and their reaction with acids.
The s-block elements, which include the alkali metals (Group 1) and alkaline earth metals (Group 2), are highly reactive metals that readily react with acids to form salts and release hydrogen gas. The general reaction between an s-block metal and an acid can be represented as:
M + HX → MX + H2
where M represents an s-block metal, HX represents an acid, MX represents a salt, and H2 represents hydrogen gas.
The reactivity of s-block metals with acids increases as you move down the group, meaning that the reaction becomes more vigorous and releases more hydrogen gas. For example, lithium (Li) is the least reactive alkali metal and reacts slowly with acids, while francium (Fr) is the most reactive and reacts violently with acids.
In summary, s-block elements readily react with acids to form salts and hydrogen gas, with the reactivity increasing as you move down the group.
Acid

A corrosive is a substance that can give a hydrogen particle (H+) (as a rule, will be a proton) to another substance. Acids have a pH under 7.0. A synthetic can give a proton in the event that the hydrogen iota is joined to an electronegative molecule like oxygen, nitrogen, or chlorine. A few acids are solid and others are powerless. The feeble acids clutch a portion of their protons, while the solid acids let go of every one of them. All acids will deliver hydrogen particles into arrangements. How much particles that get delivered per atom will decide whether the corrosive is feeble or solid. Powerless acids will be acids that to some extent discharge the hydrogen iotas that are connected. These acids, then, at that point, may bring down pH by separation of hydrogen particles, however not totally. Powerless acids by and large have a pH worth of 4-6 while solid acids have a pH worth of 1 to 3.
A base is a corrosive’s “compound inverse.” A base is a substance that will acknowledge the corrosive’s hydrogen molecule. Bases are particles that can part separated in water and delivery hydroxide particles.
How acids work

Acids and bases commonly exist together in harmony. This intends that inside an example of a corrosive, a few particles will surrender their protons and others will acknowledge them. Indeed, even water is a combination of an acidic particle, H3O+ (called a hydronium particle) and a fundamental particle, Goodness (called a hydroxide particle). A hydronium particle will surrender its proton to a hydroxide particle, framing two atoms of H2O, which is unbiased. This response happens persistently in an example of water, yet generally the example is unbiased on the grounds that there are equivalent measures of hydronium and hydroxide in the example. For most responses, be that as it may, the acids and bases are absent in equivalent sums, and this irregularity permits a synthetic response to happen.
Each corrosive has a form base framed by eliminating the corrosive’s proton. Hydrochloric corrosive (HCl), for instance, is a corrosive and its form base is a chlorine anion, or Cl-. A corrosive and its form base are inverse in strength. Since HCl is areas of strength for a, Cl-is a feeble base.
Acid–base reaction
A corrosive base response is a substance response that happens between a corrosive and a base. It tends to be utilized to decide pH through titration. A few hypothetical structures give elective originations of the response components and their application in taking care of related issues; these are known as the corrosive base hypotheses, for instance, Brønsted-Lowry corrosive base hypothesis.
Their significance becomes clear in dissecting corrosive base responses for vaporous or fluid species, or when corrosive or base person might be fairly less evident. The first of these ideas was given by the French scientific expert Antoine Lavoisier, around 1776.
It is essential to consider the corrosive base response models as hypotheses that complete one another. For instance, the ongoing Lewis model has the broadest meaning of what a corrosive and base are, with the Brønsted-Lowry hypothesis being a subset of what acids and bases are, and the Arrhenius hypothesis being the most prohibitive.
Amino acid
Amino acids are natural mixtures that contain both amino and carboxylic corrosive practical gatherings. Albeit more than 500 amino acids exist in nature, by a long shot the most significant are the alpha-amino acids, which contain proteins. Just 22 alpha amino acids show up in the hereditary code.
Amino acids can be arranged by the areas of the center primary utilitarian gatherings, as alpha-(α-), beta-(β-), gamma-(γ-) or delta-(δ-) amino acids; different classifications connect with extremity, ionization, and side chain bunch type (aliphatic, non-cyclic, fragrant, containing hydroxyl or sulfur, and so on.). As proteins, amino corrosive deposits structure the second-biggest part (water being the biggest) of human muscles and different tissues. Past their job as deposits in proteins, amino acids take part in various cycles, for example, synapse transport and biosynthesis. It is believed that they assumed a vital part in empowering life on The planet and its rise.
Amino acids are officially named by the IUPAC-IUBMB Joint Commission based on Biochemical Terminology in conditions of the imaginary “nonpartisan” structure displayed in the delineation. For instance, the precise name of alanine is 2-aminopropanoic corrosive, in view of the equation CH3−CH(NH2)−COOH. The Commission supported this methodology as follows:
The methodical names and equations given allude to speculative structures in which amino gatherings are unprotonated and carboxyl gatherings are undissociated. This show is valuable to keep away from different nomenclatural issues however ought not be taken to suggest that these designs address a considerable part of the amino-corrosive atoms.
Case Study on Acids
Fertilizers are chemicals that are added to soil to promote plant growth and increase crop yield. One of the most commonly used fertilizers is ammonium phosphate, which is produced by reacting ammonia (NH3) with phosphoric acid (H3PO4). This reaction produces ammonium phosphate (NH4)3PO4, a salt that contains both nitrogen and phosphorus, two essential nutrients for plant growth.
The process of producing ammonium phosphate fertilizer begins with the extraction of phosphate rock from mines. The rock is then ground into a fine powder and treated with sulfuric acid (H2SO4) to produce phosphoric acid (H3PO4). This reaction is known as the wet process, and it produces a mixture of phosphoric acid and calcium sulfate (CaSO4) called phosphogypsum. The phosphoric acid is then purified by removing impurities such as fluoride, arsenic, and heavy metals.
The purified phosphoric acid is then reacted with ammonia gas (NH3) in a reactor vessel to produce ammonium phosphate [(NH4)3PO4]. The reaction is exothermic, meaning that it releases heat, so the reactor vessel must be carefully controlled to prevent overheating. The resulting ammonium phosphate solution is then dried and granulated to produce the final fertilizer product.
The use of acids in the production of fertilizers is a crucial step in agriculture and helps to increase crop yields and improve food security. However, the production of fertilizers can also have negative environmental impacts, such as the release of greenhouse gases during the production process and the contamination of waterways with excess nutrients from fertilizer runoff. Therefore, it is important to carefully manage the production and use of fertilizers to minimize these impacts.
White paper on Acids
Introduction:
Acids are a class of chemical compounds that have unique properties and play important roles in various chemical reactions and biological processes. They are characterized by their ability to donate hydrogen ions (H+) when dissolved in water, giving them a sour taste and the ability to react with certain metals and bases.
Types of Acids:
Acids can be classified as either strong or weak, depending on their ability to dissociate in water. Strong acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), dissociate completely in water to produce a high concentration of hydrogen ions. Weak acids, such as acetic acid (CH3COOH) and citric acid (C6H8O7), only partially dissociate in water, resulting in a lower concentration of hydrogen ions.
Properties of Acids:
Acids have several properties that make them unique and useful in various applications. Some of these properties include:
- Sour taste: Acids have a sour taste, which is why they are often used in food and beverage industries as flavor enhancers and preservatives.
- Reactivity with metals: Acids can react with certain metals to produce hydrogen gas, which is used in various applications such as welding and fuel cells.
- Reactivity with bases: Acids react with bases to form salts and water, a process known as neutralization. This reaction is commonly used in the production of various chemicals, including fertilizers and pharmaceuticals.
- pH: Acids have a low pH, typically less than 7 on the pH scale, and can be used to adjust the pH of various solutions.
Applications of Acids:
Acids have a wide range of applications in various industries, including:
- Chemical industry: Acids are used in the production of various chemicals, such as fertilizers, dyes, and pharmaceuticals.
- Food industry: Acids are used as preservatives and flavor enhancers in various food and beverage products.
- Cleaning industry: Acids, such as hydrochloric acid and sulfuric acid, are used in cleaning products to remove stains and other contaminants from surfaces.
- Medical industry: Acids, such as citric acid and ascorbic acid, are used in the production of various medications and supplements.
Safety Considerations:
While acids have many useful applications, they can also be hazardous if not handled properly. Acids are corrosive and can cause chemical burns if they come into contact with skin or eyes. Proper safety equipment, such as gloves, goggles, and lab coats, should be worn when working with acids. Acids should also be stored in appropriate containers and handled with care to prevent spills and other accidents.
Conclusion:
Acids are a diverse class of chemical compounds that have unique properties and play important roles in various industries and applications. While they can be hazardous if not handled properly, their many useful applications make them an important part of modern society. Understanding the properties and applications of acids is essential for scientists, engineers, and professionals working in various industries.
