Acids and bases are fundamental concepts in chemistry that are used to describe the properties of various chemical substances. The Bronsted and Lewis concepts are two different approaches used to define acids and bases.
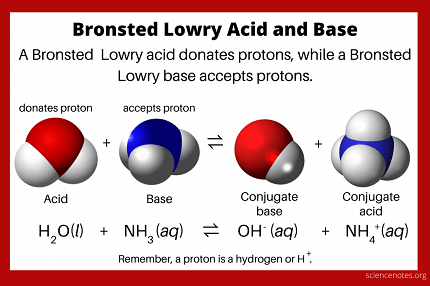
The Bronsted-Lowry concept defines an acid as a substance that donates a proton (H+) and a base as a substance that accepts a proton. In other words, acids are proton donors and bases are proton acceptors. For example, in the reaction HCl + H2O -> H3O+ + Cl-, HCl is the acid because it donates a proton to water (H2O), which is acting as a base by accepting the proton to form a hydronium ion (H3O+).
The Lewis concept defines an acid as a substance that accepts an electron pair and a base as a substance that donates an electron pair. This definition is more general and applies to reactions involving elements other than hydrogen. For example, in the reaction AlCl3 + Cl- -> AlCl4-, AlCl3 is the acid because it accepts a pair of electrons from chloride ion (Cl-), which is acting as a base by donating the electron pair to form an AlCl4- ion.
It is important to note that not all acids and bases fit perfectly into either the Bronsted or Lewis definitions, and sometimes a substance can be both an acid and a base depending on the context in which it is used.
What is Required Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
In chemical and ionic equilibrium, acids and bases play a crucial role. The Bronsted and Lewis concepts are used to define and understand the behavior of acids and bases in equilibrium systems.
In the Bronsted concept, an acid is a proton (H+) donor, and a base is a proton acceptor. In an equilibrium system, the concentration of protons (H+) and hydroxide ions (OH-) determines the pH of the system. If the concentration of H+ is greater than the concentration of OH-, the solution is acidic, and if the concentration of OH- is greater than the concentration of H+, the solution is basic. In an equilibrium system, an acid will donate a proton to a base, forming a conjugate base and a conjugate acid. The strength of an acid is determined by its ability to donate protons, and the strength of a base is determined by its ability to accept protons.
In the Lewis concept, an acid is an electron acceptor, and a base is an electron donor. In an equilibrium system, an acid will accept an electron pair from a base, forming a coordinate covalent bond. The strength of an acid is determined by its ability to accept electron pairs, and the strength of a base is determined by its ability to donate electron pairs.
In chemical and ionic equilibrium, the behavior of acids and bases is critical to maintaining the stability of the system. Understanding the Bronsted and Lewis concepts of acids and bases allows us to predict and manipulate the behavior of these species in equilibrium systems.
When is Required Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
The concepts of acids and bases and chemical/ionic equilibrium are important in many areas of chemistry, including analytical chemistry, biochemistry, physical chemistry, and environmental chemistry. They are relevant in any situation where chemical reactions occur, and the behavior of acidic and basic species plays a role in the system’s equilibrium.
Examples of when these concepts are important include:
- In biological systems, such as the pH of blood or the ionization of amino acids in proteins.
- In industrial chemical processes, such as the production of fertilizers or the synthesis of pharmaceuticals.
- In environmental chemistry, such as the buffering capacity of soil or the acidification of water bodies due to acid rain.
- In analytical chemistry, such as determining the acidity or basicity of a solution through titration or pH measurements.
In summary, the concepts of acids and bases and chemical/ionic equilibrium are important in a wide range of contexts in chemistry and are relevant whenever chemical reactions occur.
Where is Required Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
The concepts of acids and bases and chemical/ionic equilibrium are relevant in various fields of chemistry, including:
- Analytical chemistry: In analytical chemistry, the Bronsted and Lewis concepts of acids and bases are used to understand and analyze the behavior of acidic and basic species in solution. For example, titration is a common technique used to determine the acidity or basicity of a solution by reacting it with a known concentration of acid or base.
- Biochemistry: In biochemistry, the Bronsted and Lewis concepts of acids and bases are essential to understanding the behavior of amino acids, proteins, and enzymes. The pH of biological fluids, such as blood and urine, is regulated by the presence of buffering systems that help maintain a constant pH.
- Environmental chemistry: In environmental chemistry, the concepts of acids and bases are used to understand the impact of acid rain on soil and water bodies. The buffering capacity of soil is important in agriculture, as it helps to regulate the pH of the soil and can affect crop yields.
- Physical chemistry: In physical chemistry, the Bronsted and Lewis concepts of acids and bases are used to understand the properties of solutions and the behavior of chemical reactions. The equilibrium constant (Kc) is a measure of the degree to which a reaction has reached equilibrium, and it is dependent on the concentrations of acidic and basic species.
In summary, the concepts of acids and bases and chemical/ionic equilibrium are relevant in various fields of chemistry, including analytical chemistry, biochemistry, environmental chemistry, and physical chemistry.
How is Required Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
The concepts of acids and bases and chemical/ionic equilibrium are interrelated, and understanding their relationship is important in many areas of chemistry.
The Bronsted concept of acids and bases defines an acid as a proton (H+) donor and a base as a proton acceptor. In an equilibrium system, an acid will donate a proton to a base, forming a conjugate base and a conjugate acid. The strength of an acid is determined by its ability to donate protons, and the strength of a base is determined by its ability to accept protons.
In chemical/ionic equilibrium, the concentration of acidic and basic species in a solution determines the pH of the system. The pH of a solution is a measure of its acidity or basicity and is defined as the negative logarithm of the concentration of H+ ions in the solution. In an equilibrium system, the concentration of H+ and OH- ions are related by the ion product constant (Kw), which is a constant for a given temperature.
The Lewis concept of acids and bases defines an acid as an electron acceptor and a base as an electron donor. In an equilibrium system, an acid will accept an electron pair from a base, forming a coordinate covalent bond. The strength of an acid is determined by its ability to accept electron pairs, and the strength of a base is determined by its ability to donate electron pairs.
In summary, the concepts of acids and bases and chemical/ionic equilibrium are interrelated. The Bronsted and Lewis concepts help us understand the behavior of acidic and basic species in an equilibrium system, while chemical/ionic equilibrium helps us understand the relationship between the concentration of H+ and OH- ions and the pH of a solution.
Nomenclature of Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
Nomenclature of acids and bases refers to the naming conventions used to identify these substances based on their properties and composition. The naming conventions are based on the Bronsted and Lewis concepts of acids and bases and are used to indicate the strength and behavior of acidic and basic substances. In addition, nomenclature is also used in chemical and ionic equilibrium to describe the equilibrium state of a solution.
Acid Nomenclature: The Bronsted-Lowry theory provides the basis for the nomenclature of acids. According to this theory, an acid is a substance that donates protons (H+) in a chemical reaction. Acids can be classified as strong or weak, depending on their ability to donate protons. Strong acids are those that completely dissociate in water, while weak acids only partially dissociate. The nomenclature of acids follows the format: “prefix + acid.”
For example, hydrochloric acid (HCl) is a strong acid that completely dissociates in water, while acetic acid (CH3COOH) is a weak acid that only partially dissociates. The prefix “hydro-” is used for binary acids (composed of hydrogen and one other element) and the suffix “-ic” is used for the anion of the acid. In the case of acetic acid, the prefix “acet-” is used, followed by the suffix “-ic.”
Base Nomenclature: The Lewis theory of acids and bases provides the basis for the nomenclature of bases. According to this theory, a base is a substance that donates an electron pair in a chemical reaction. Bases can also be classified as strong or weak, depending on their ability to donate electron pairs. The nomenclature of bases follows the format: “metal name or positive ion + hydroxide.”
For example, sodium hydroxide (NaOH) and potassium hydroxide (KOH) are strong bases that completely dissociate in water, while ammonia (NH3) is a weak base that only partially dissociates. In the case of sodium hydroxide and potassium hydroxide, the metal name (sodium or potassium) is followed by the word “hydroxide.” In the case of ammonia, the name remains as “ammonia,” and the term “base” is added to indicate its basic nature.
Chemical and Ionic Equilibrium Nomenclature: In chemical and ionic equilibrium, nomenclature is used to describe the state of the equilibrium. The equilibrium constant, Keq, is used to quantify the extent of the reaction, and the direction of the reaction can be determined based on the value of Keq. A large Keq value indicates that the reaction strongly favors the products, while a small Keq value indicates that the reaction strongly favors the reactants.
For example, the equilibrium constant for the dissociation of acetic acid in water is given by the expression: CH3COOH + H2O ⇌ CH3COO- + H3O+. The equilibrium constant expression is written as Keq = [CH3COO-][H3O+]/[CH3COOH][H2O]. A large Keq value for this reaction indicates that the dissociation of acetic acid strongly favors the products, which are acetate ions and hydronium ions.
Conclusion: The nomenclature of acids and bases and chemical/ionic equilibrium provides a systematic way to identify and describe these substances based on their properties and behavior. The Bronsted-Lowry theory provides the basis for acid nomenclature, while the Lewis theory provides the basis for base nomenclature. Chemical and ionic equilibrium nomenclature is used to describe the state of an equilibrium and provides a way to quantify the extent and direction of a chemical reaction.
Case Study on Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
Case Study: The Effect of pH on Enzyme Activity
Enzymes are proteins that catalyze biochemical reactions in living organisms. They are essential for many biological processes, including metabolism, digestion, and DNA replication. The activity of enzymes is affected by many factors, including pH. In this case study, we will examine how changes in pH affect the activity of the enzyme catalase.
Catalase is an enzyme found in all living organisms that catalyzes the breakdown of hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2). The reaction is as follows:
2H2O2 → 2H2O + O2
Catalase is active over a wide range of pH values, but its activity is optimal at a pH of around 7.0. In this pH range, the active site of the enzyme has the correct shape to interact with the substrate (H2O2) and catalyze the reaction.
If the pH of the solution is changed, the shape of the active site can be altered, which can affect the enzyme’s activity. At pH values below 7.0, the enzyme becomes less active because the H+ ions in the solution interfere with the interaction between the enzyme and the substrate. At pH values above 7.0, the enzyme also becomes less active because the OH- ions in the solution interfere with the interaction between the enzyme and the substrate.
To investigate the effect of pH on catalase activity, a researcher could perform an experiment as follows:
- Obtain a sample of catalase and a sample of hydrogen peroxide.
- Prepare a series of solutions with different pH values (e.g., pH 4, 5, 6, 7, 8, 9, and 10) using buffer solutions.
- Add a fixed amount of catalase to each solution.
- Add a fixed amount of hydrogen peroxide to each solution.
- Measure the rate of oxygen production in each solution over a fixed period of time (e.g., 5 minutes).
- Plot the rate of oxygen production (y-axis) against the pH of the solution (x-axis).
The results of this experiment would show that catalase activity is optimal at a pH of around 7.0 and decreases at pH values below or above this value. The data could be analyzed using the Bronsted and Lewis concepts of acids and bases and chemical/ionic equilibrium to understand the underlying chemical processes involved.
Overall, this case study demonstrates how the concepts of acids and bases and chemical/ionic equilibrium are important in understanding the behavior of enzymes and the factors that affect their activity.
White paper on Acids and bases (Bronsted and Lewis concepts) Chemical and Ionic Equilibrium
Title: Understanding Acids and Bases: Bronsted and Lewis Concepts and Chemical/Ionic Equilibrium
Introduction:
Acids and bases are fundamental concepts in chemistry and play crucial roles in many chemical reactions and biological processes. Two main theories of acids and bases, the Bronsted-Lowry theory and the Lewis theory, provide different perspectives on the nature of acid-base reactions. Additionally, chemical and ionic equilibrium provides a framework for understanding the behavior of acidic and basic species in a solution. This white paper will explore these concepts in-depth and highlight their practical applications.
Bronsted-Lowry Theory:
According to the Bronsted-Lowry theory, an acid is a proton (H+) donor, and a base is a proton acceptor. When an acid donates a proton, it forms a conjugate base, and when a base accepts a proton, it forms a conjugate acid. The strength of an acid is determined by its ability to donate protons, and the strength of a base is determined by its ability to accept protons. The acid dissociation constant, Ka, is used to quantify the strength of an acid. The higher the value of Ka, the stronger the acid.
Lewis Theory:
The Lewis theory of acids and bases defines an acid as an electron pair acceptor and a base as an electron pair donor. In this theory, the focus is on the behavior of electron pairs rather than protons. The strength of an acid is determined by its ability to accept electron pairs, and the strength of a base is determined by its ability to donate electron pairs. The Lewis theory of acids and bases can be used to describe reactions involving compounds that do not contain protons, such as metal ions and non-metallic compounds.
Chemical and Ionic Equilibrium:
Acids and bases are involved in many equilibrium reactions, where the forward and reverse reactions occur at the same rate, resulting in a stable system. In chemical equilibrium, the concentrations of reactants and products are constant, and the equilibrium constant, Keq, can be used to quantify the extent of the reaction. In ionic equilibrium, the concentrations of H+ and OH- ions in a solution determine the pH of the system. The pH of a solution is a measure of its acidity or basicity and is defined as the negative logarithm of the concentration of H+ ions in the solution.
Applications:
The concepts of acids and bases and chemical/ionic equilibrium are widely used in many areas of chemistry. For example, they are essential in understanding the behavior of enzymes and the factors that affect their activity, as seen in the case study presented earlier. They are also used in acid-base titration experiments, where a solution of known concentration of an acid or base is used to determine the concentration of an unknown solution. In addition, they are important in understanding the behavior of natural and synthetic polymers, as their acidic and basic properties can affect their solubility, reactivity, and mechanical properties.
Conclusion:
In summary, the concepts of acids and bases and chemical/ionic equilibrium provide a fundamental understanding of the behavior of acidic and basic species in chemical systems. The Bronsted-Lowry theory and the Lewis theory provide complementary perspectives on acid-base reactions, while chemical and ionic equilibrium provides a framework for understanding the behavior of these species in a solution. These concepts have many practical applications in chemistry and are essential for understanding the behavior of biological systems, natural and synthetic polymers, and many other chemical processes.
