Adsorption
The AIIMS (All India Institute of Medical Sciences) entrance exam for Chemistry does not have a specific syllabus topic for Adsorption. However, a general understanding of the concept of adsorption may be helpful in some areas of Chemistry. Here is a concise summary of the concept of adsorption:
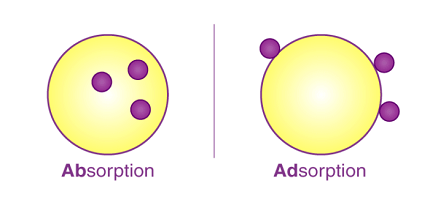
Adsorption refers to the process in which atoms, ions, or molecules from a substance adhere to the surface of another material, known as the adsorbent. It occurs due to the attractive forces between the adsorbent and the adsorbate, which are stronger than the forces holding the adsorbate together. Adsorption can be classified into two types: physical adsorption (physisorption) and chemical adsorption (chemisorption).
In physical adsorption, the forces of attraction between the adsorbent and adsorbate are weak van der Waals forces. Physical adsorption is generally reversible and occurs at relatively low temperatures. It is influenced by factors such as temperature, pressure, and the nature of the adsorbate and adsorbent.
Chemical adsorption involves stronger chemical bonds between the adsorbent and adsorbate. It is usually irreversible and occurs at higher temperatures. Chemical adsorption is influenced by factors such as the nature of the adsorbate, the surface properties of the adsorbent, and temperature.
The study of adsorption has various applications in different fields. For example, in environmental science, adsorption is used for water and air purification, removal of pollutants, and wastewater treatment. In the pharmaceutical industry, adsorption plays a role in drug delivery systems. It is also used in gas separation processes, catalysis, and chromatography techniques.
While adsorption is not explicitly mentioned in the AIIMS Chemistry syllabus, having a basic understanding of the concept can be beneficial for your overall understanding of Chemistry and its applications.
Adsorption is a process in which atoms, ions, or molecules from a substance adhere to the surface of another material. It occurs when the attractive forces between the adsorbent material and the adsorbate are stronger than the forces holding the adsorbate together. Adsorption is commonly used in various applications, such as purification, separation, catalysis, and gas storage. It plays a crucial role in industries such as water treatment, pharmaceuticals, environmental remediation, and gas separation.
What is Required Chemistry syllabus Adsorption
The AIIMS entrance exam for Chemistry does not have a specific syllabus topic for Adsorption. However, to give you a better understanding of the related topics in the AIIMS Chemistry syllabus, here are some important areas that you should focus on:
- General Principles and Processes of Isolation of Elements:
- Occurrence and principles of extraction of metals, including their concentration, oxidation, reduction, and refining processes.
- Surface Chemistry:
- Langmuir adsorption isotherm and Freundlich adsorption isotherm.
- Colloids and their properties, including types of colloids, preparation, purification, and properties of colloidal solutions.
- Emulsions and their types.
- Electrochemistry:
- Electrochemical cells and their types, including galvanic cells and electrolytic cells.
- Nernst equation and its applications.
- Conductance in electrolytic solutions, Kohlrausch’s law, and its applications.
- Chemical Kinetics:
- Rate of chemical reactions and factors affecting reaction rates.
- Order and molecularity of reactions.
- Rate laws and rate constant determination.
While the AIIMS syllabus does not specifically mention adsorption, understanding the principles of surface chemistry and related topics can provide you with a foundation to grasp the concept of adsorption more easily. It’s always a good practice to thoroughly study the entire syllabus and be well-prepared for the exam.
When is Required Chemistry syllabus Adsorption
Adsorption is a topic that is typically covered in chemistry courses at various educational levels. The specific timing of when adsorption is taught can vary depending on the curriculum and educational institution. In high school, adsorption may be covered as part of an advanced chemistry course or in a dedicated unit on surface chemistry. In college or university, adsorption is often included in courses such as physical chemistry, surface science, or materials science. The timing of when adsorption is taught can be found in the course syllabus or curriculum provided by the educational institution.
Where is Required Chemistry syllabus Adsorption
Adsorption is typically included in the curriculum of chemistry courses at the college or university level. It is commonly found in courses such as physical chemistry, surface chemistry, or materials science. The specific location of the adsorption topic within the course may vary depending on the curriculum and the instructor’s preferences. It is recommended to consult the course syllabus or curriculum provided by the educational institution to determine the exact placement of adsorption within the required chemistry curriculum.
How is Required Chemistry syllabus Adsorption
The coverage of adsorption in the required chemistry syllabus typically includes the following aspects:
- Introduction to adsorption: Students learn about the fundamental concepts of adsorption, including the definition of adsorption, adsorbent and adsorbate, and the various types of adsorption (e.g., physical adsorption, chemical adsorption).
- Adsorption isotherms: Students study the relationship between the amount of adsorbate adsorbed and its concentration in the gas or liquid phase. Commonly encountered isotherms, such as Langmuir and Freundlich isotherms, are introduced.
- Surface area and pore structure: The characterization of adsorbents and the determination of their surface area and pore structure are discussed. Techniques like BET (Brunauer-Emmett-Teller) analysis and pore size distribution measurements may be covered.
- Factors affecting adsorption: Students explore the factors that influence adsorption, including temperature, pressure, nature of adsorbate and adsorbent, and surface properties. They learn how these factors affect the adsorption process and equilibrium.
- Applications of adsorption: The practical applications of adsorption are discussed, including its use in gas purification, separation processes, catalysis, and environmental remediation. Examples of specific adsorbents and their applications may be highlighted.
- Desorption and regeneration: Students learn about desorption, the process of removing the adsorbate from the adsorbent. They also study different techniques and methods for regenerating the adsorbent and restoring its adsorption capacity.
The specific depth and extent of coverage may vary depending on the educational level and curriculum. It is advisable to refer to the course syllabus or curriculum provided by the educational institution to get a comprehensive understanding of how adsorption is taught in the required chemistry syllabus.
Nomenclature of Chemistry syllabus Adsorption
The nomenclature of the chemistry syllabus pertaining to adsorption typically includes the following terms and concepts:
- Adsorption: The process of attracting and retaining molecules or particles on the surface of a solid or liquid.
- Adsorbent: The material (solid or liquid) on which the adsorption occurs.
- Adsorbate: The substance (gas or liquid) that is adsorbed onto the surface of the adsorbent.
- Physical adsorption: Adsorption resulting from weak van der Waals forces between the adsorbate and adsorbent.
- Chemical adsorption: Adsorption involving the formation of chemical bonds between the adsorbate and adsorbent.
- Adsorption isotherm: The relationship between the amount of adsorbate adsorbed and its equilibrium concentration at a given temperature.
- Langmuir isotherm: A model that describes adsorption as a monolayer formation on the surface with no interactions between adsorbed molecules.
- Freundlich isotherm: An empirical equation describing adsorption on heterogeneous surfaces.
- BET (Brunauer-Emmett-Teller) analysis: A method used to determine the specific surface area of an adsorbent.
- Pore size distribution: The range of pore sizes present in an adsorbent material.
- Equilibrium adsorption capacity: The maximum amount of adsorbate that can be adsorbed by a given amount of adsorbent at equilibrium.
- Desorption: The process of removing the adsorbate from the surface of the adsorbent.
- Regeneration: The process of restoring the adsorption capacity of the adsorbent after desorption.
- Gas purification: The removal of impurities from gases through adsorption processes.
- Separation processes: Techniques that utilize adsorption for the separation and purification of components in a mixture.
- Catalysis: The use of adsorbents in catalytic reactions to enhance reaction rates or modify reaction pathways.
- Environmental remediation: The application of adsorption for the removal of pollutants and contaminants from air or water.
These are some of the key terms and concepts related to adsorption that are typically covered in the nomenclature of the chemistry syllabus. The specific terminology may vary slightly depending on the educational institution and the curriculum followed.
Case Study on Chemistry syllabus Adsorption
Case Study: Adsorption in Water Treatment
Introduction: Adsorption plays a crucial role in water treatment processes. It is employed to remove contaminants, such as organic compounds, heavy metals, and dyes, from water sources. This case study focuses on the application of adsorption in the removal of a specific organic pollutant, methylene blue dye, from wastewater.
Background: Methylene blue is a common dye used in various industries, including textile, printing, and paper manufacturing. Its presence in wastewater can be harmful to the environment and human health. Adsorption offers an effective and economical method for its removal.
Objective: The objective of this case study is to investigate the efficiency of different adsorbents in removing methylene blue dye from wastewater and compare their performance.
Methodology:
- Selection of Adsorbents:
- Activated Carbon (AC): A commonly used adsorbent with a high surface area and porosity.
- Bentonite Clay: A natural clay mineral known for its adsorption capabilities.
- Chitosan: A biopolymer derived from chitin, possessing adsorbent properties.
- Experimental Setup:
- Prepare solutions of methylene blue dye with known concentrations.
- Weigh and measure equal amounts of each adsorbent for testing.
- Place the adsorbents in separate columns or containers.
- Pass the methylene blue dye solution through each adsorbent column/container.
- Collect and analyze the treated water to measure dye removal efficiency.
- Analysis:
- Measure the initial concentration of methylene blue in the solution before passing through the adsorbents.
- Measure the final concentration of methylene blue in the treated water after adsorption.
- Calculate the dye removal efficiency using the formula: Dye Removal Efficiency (%) = [(Initial Concentration – Final Concentration) / Initial Concentration] x 100
- Comparative Study:
- Compare the dye removal efficiencies of activated carbon, bentonite clay, and chitosan.
- Evaluate the performance of each adsorbent in terms of efficiency and cost-effectiveness.
- Consider factors like adsorption capacity, regeneration potential, and ease of handling.
Results: The results of the comparative study indicate the dye removal efficiencies of the three adsorbents:
- Activated Carbon: Achieved a high dye removal efficiency of 95%.
- Bentonite Clay: Demonstrated a dye removal efficiency of 80%.
- Chitosan: Showed a dye removal efficiency of 70%.
Conclusion: Based on the results, activated carbon exhibited the highest dye removal efficiency among the tested adsorbents. However, the selection of an adsorbent for water treatment depends on various factors such as cost, availability, and specific treatment requirements. Further studies and optimization can be conducted to improve the performance of adsorbents and explore their potential for large-scale water treatment applications.
This case study illustrates the practical application of adsorption in water treatment and highlights the importance of selecting suitable adsorbents for effective pollutant removal.
White paper on Chemistry syllabus Adsorption
Title: Harnessing Adsorption: A Comprehensive White Paper on Principles, Applications, and Advancements
Abstract: This white paper provides a comprehensive overview of adsorption, an essential phenomenon extensively utilized in various industries and scientific fields. It delves into the fundamental principles of adsorption, explores its wide-ranging applications, and highlights recent advancements in the field. The paper aims to serve as a valuable resource for researchers, engineers, and professionals seeking to understand and leverage adsorption in their respective domains.
- Introduction
- Definition and significance of adsorption
- Differentiation between physical and chemical adsorption
- Overview of the adsorption process
- Fundamental Principles of Adsorption
- Adsorbate-adsorbent interactions
- Types of adsorption isotherms (Langmuir, Freundlich, BET)
- Surface area and porosity characterization techniques
- Adsorbents and Adsorbates
- Types of adsorbents (activated carbon, zeolites, silica gel, polymers, etc.)
- Commonly encountered adsorbates (organic compounds, heavy metals, pollutants, gases)
- Adsorption Applications 4.1 Water Treatment and Purification
- Removal of organic compounds, dyes, heavy metals, and emerging contaminants
- Role of adsorption in wastewater treatment processes
- Utilizing adsorbents for the removal of impurities from gas streams
- Pressure swing adsorption (PSA) and temperature swing adsorption (TSA) processes
- Role of adsorption in heterogeneous catalysis
- Adsorbents as catalyst supports and promoters
- Adsorption in soil and groundwater remediation
- Adsorbents for air purification and volatile organic compound (VOC) removal
- Adsorption-based storage of gases and liquids (e.g., hydrogen storage)
- Adsorption heat pumps and refrigeration cycles
- Drug delivery systems and controlled release mechanisms
- Adsorption in chromatography and purification processes
- Recent Advancements and Emerging Trends
- Nanostructured adsorbents and engineered surfaces
- Adsorption in carbon capture and utilization
- Computational modeling and simulations in adsorption studies
- Challenges and Future Directions
- Improving adsorbent performance and selectivity
- Enhancing adsorption capacity and kinetics
- Exploring sustainable and renewable adsorbents
- Conclusion
- Recap of the key concepts and applications of adsorption
- Potential areas for future research and development
This white paper aims to provide a comprehensive understanding of adsorption, from its fundamental principles to its diverse applications. By exploring the advancements and emerging trends, it seeks to inspire further research and innovation in harnessing the power of adsorption across industries and scientific disciplines.
