Colloid properties
The syllabus for the Chemistry section of the AIIMS (All India Institute of Medical Sciences) entrance exam may vary from year to year, and I don’t have access to the most up-to-date syllabus. However, I can provide you with a general overview of colloid properties, which are often covered in the Chemistry syllabus for medical entrance exams.
Colloids are a type of mixture in which particles of one substance are dispersed evenly throughout another substance. They consist of two components: a dispersed phase (the particles) and a dispersing medium (the substance in which the particles are dispersed). Here are some important properties of colloids:
- Particle Size: Colloidal particles are larger than molecules but smaller than those in a suspension. They typically have a size range of 1-1000 nanometers.
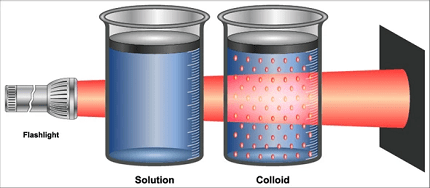
- Tyndall Effect: When a beam of light passes through a colloid, it scatters the light, making the path of the light visible. This phenomenon is known as the Tyndall effect and is used to distinguish between true solutions and colloidal dispersions.
- Brownian Motion: Colloidal particles exhibit random, zigzag motion due to collisions with the molecules of the dispersing medium. This motion is called Brownian motion and is caused by the kinetic energy of the particles.
- Stability: Colloidal systems can be stable or unstable. Stable colloids resist the tendency to settle down or coagulate, while unstable colloids tend to aggregate and separate. Stability can be influenced by factors such as particle charge, repulsion between particles, and presence of stabilizing agents.
- Electrophoresis: Colloidal particles may migrate under the influence of an electric field. This phenomenon is known as electrophoresis and is used for the separation and characterization of colloidal particles.
- Coagulation and Peptization: Coagulation refers to the aggregation and settling of colloidal particles, leading to the formation of a precipitate. On the other hand, peptization is the process of converting a precipitate back into a stable colloid by the addition of a suitable dispersing medium or stabilizing agent.
- Surface Tension: Colloidal particles at the interface between two immiscible phases can affect the surface tension of the system. This property plays a role in emulsion formation and stability.
It is important to note that the syllabus for the AIIMS entrance exam may cover additional topics related to colloid properties, and it’s recommended to refer to the official syllabus or study materials provided by AIIMS for the most accurate and up-to-date information.
What is Required AIIMS-SYLLABUS Chemistry syllabus Colloid properties
Typically, the Chemistry syllabus for AIIMS covers topics from the NCERT (National Council of Educational Research and Training) curriculum up to the 12th grade level. Colloid properties are often included in the broader topic of “Solutions and Colloidal State.” The syllabus may include concepts such as:
- Types of solutions: True solutions, colloidal solutions, and suspensions.
- Tyndall effect and its applications.
- Brownian motion and its significance.
- Coagulation and peptization processes.
- Emulsions and their properties.
- Micelles and their formation.
- Surface chemistry related to colloids.
Please note that this is a general overview, and the specific subtopics and depth of coverage may vary. It is highly recommended to consult the official AIIMS website or the relevant exam notification for the most accurate and detailed syllabus information.
How is Required AIIMS-SYLLABUS Chemistry syllabus Colloid properties
Colloid properties refer to the characteristics and behaviors of colloidal systems, which are mixtures in which particles of one substance are dispersed throughout another substance. Here are some key properties of colloids:
- Particle Size: Colloidal particles have intermediate sizes between individual molecules and larger particles found in suspensions. They typically range in size from 1 nanometer to 1 micrometer. The small size of colloidal particles contributes to their unique properties and behavior.
- Tyndall Effect: When a beam of light passes through a colloid, the light is scattered by the dispersed particles. This scattering of light is known as the Tyndall effect and can be observed as a visible beam or halo of light in the colloid. The Tyndall effect is used to identify the presence of colloidal particles and distinguish colloids from true solutions.
- Brownian Motion: Colloidal particles exhibit random and continuous motion, known as Brownian motion. This motion is caused by the constant collision of the particles with the molecules of the dispersing medium. Brownian motion prevents the settling of colloidal particles and helps keep the colloid dispersed.
- Surface Area and Surface Charge: Colloidal particles have a relatively large surface area compared to their volume. This increased surface area allows for interactions with the surrounding medium and other particles, influencing the colloidal properties. Additionally, colloidal particles may carry an electrical charge on their surface, resulting in electrostatic repulsion or attraction between particles and affecting their stability.
- Stability: Colloidal systems can exhibit varying degrees of stability. Stable colloids remain dispersed and do not settle or aggregate over time. The stability of a colloid can be influenced by factors such as the size and charge of the particles, the nature of the dispersing medium, and the presence of stabilizing agents.
- Coagulation and Flocculation: Colloidal particles can undergo coagulation or flocculation, leading to the destabilization of the colloid. Coagulation refers to the irreversible aggregation of particles, resulting in the formation of larger aggregates or precipitates. Flocculation, on the other hand, involves the reversible formation of loosely bound particle clusters or flocs. Both processes can be influenced by factors such as pH, temperature, electrolyte concentration, and the presence of flocculating agents.
Understanding the properties of colloids is important in various fields, including chemistry, biology, medicine, and materials science. The study of colloid properties helps in understanding the behavior of colloidal systems, their applications, and their interactions with other substances.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Colloid properties
Colloids and their properties play a significant role in various areas of medicine, including drug delivery systems, diagnostic techniques, and biological processes. Here are a few examples that demonstrate the relevance of colloid properties in the medical field:
- Drug Delivery Systems: Colloidal systems such as liposomes and nanoparticles are extensively used in drug delivery. The size, surface charge, and stability of these colloidal carriers can be tailored to optimize drug release, enhance bioavailability, and target specific sites in the body. Understanding the properties of colloidal drug carriers is crucial for designing effective and targeted delivery systems.
- Intravenous Fluids: Colloidal solutions are used in intravenous fluids to provide hydration and deliver essential nutrients in critical care settings. The colloidal properties of these fluids, such as osmotic pressure and particle size distribution, are carefully controlled to ensure compatibility with the human body and to maintain stable fluid balance.
- Diagnostic Techniques: Colloids find applications in diagnostic techniques like immunoassays and medical imaging. Colloidal gold nanoparticles are used as labels in immunoassays for detecting biomarkers and pathogens. Magnetic nanoparticles, another type of colloid, are employed in magnetic resonance imaging (MRI) for enhanced contrast and targeted imaging.
- Blood and Cell Substitutes: Colloidal solutions have been explored as potential substitutes for blood and cellular components. Synthetic colloids, such as albumin or starch-based colloids, have been investigated for their ability to maintain oncotic pressure and provide temporary blood volume expansion during surgical procedures or trauma.
- Biological Processes: Colloid properties are crucial for various biological processes in the human body. For example, the colloidal properties of mucus in the respiratory tract influence its ability to trap and remove foreign particles, protecting the lungs. Similarly, the colloidal properties of proteins and enzymes impact their structure, function, and interactions within biological systems.
While specific case studies illustrating the application of colloid properties in the AIIMS syllabus may not be available, the understanding of colloid properties is important in several aspects of medicine and healthcare. It helps in developing effective drug delivery systems, diagnostic tools, and understanding biological processes at the molecular level.
It’s always recommended to refer to the official AIIMS syllabus or relevant study materials provided by AIIMS for specific case studies or examples related to colloid properties as per the exam requirements.
White paper on AIIMS-SYLLABUS Chemistry syllabus Colloid properties
Title: White Paper on Colloid Properties
Abstract:
This white paper provides an in-depth exploration of colloid properties and their significance in various fields. Colloids, which are mixtures with dispersed particles in a continuous medium, exhibit unique characteristics and behaviors due to their intermediate particle size. Understanding colloid properties is essential for applications in medicine, materials science, environmental science, and many other domains. This white paper aims to provide a comprehensive overview of colloid properties, their measurement techniques, and their relevance in diverse fields.
Introduction to Colloids:
1.1 Definition and Classification
1.2 Types of Colloidal Systems
1.3 Importance of Colloid Properties
Colloid Formation and Stability:
2.1 Dispersion Forces and Surface Tension
2.2 Role of Surface Charge and Electric Double Layer
2.3 Steric and Electrostatic Stabilization
2.4 Coagulation and Flocculation
Colloid Characterization:
3.1 Particle Size Analysis
3.2 Zeta Potential Measurement
3.3 Rheological Properties
3.4 Spectroscopic Techniques
Colloid Properties and Applications:
4.1 Optical Properties and the Tyndall Effect
4.2 Brownian Motion and Diffusion
4.3 Adsorption and Catalysis
4.4 Drug Delivery Systems
4.5 Environmental Remediation
4.6 Food and Beverage Industry
4.7 Cosmetics and Personal Care Products
Case Studies:
5.1 Colloid-Based Drug Delivery Systems for Cancer Therapy
5.2 Application of Colloidal Gold Nanoparticles in Diagnostics
5.3 Colloid Stability in Environmental Remediation Processes
Challenges and Future Directions:
6.1 Colloid Stability and Aggregation
6.2 Advances in Colloid Characterization Techniques
6.3 Emerging Applications and Research Areas
Conclusion:
Summarizing the importance of understanding colloid properties and their diverse applications.
This white paper aims to provide a comprehensive overview of colloid properties, their measurement techniques, and their applications across various fields. By exploring the fundamental principles and case studies, it highlights the significance of colloid science in addressing challenges and driving advancements in medicine, materials science, and environmental remediation. Understanding colloid properties is crucial for designing innovative solutions and optimizing processes in numerous industries, making it an area of active research and exploration for scientists and engineers.
Please note that this white paper is a general outline and should be further developed and expanded with specific information, research findings, and case studies to suit the intended purpose and audience.
