General principles and processes of isolations of elements
The general principles and processes of isolating elements involve various techniques and methods used to separate elements from their compounds or natural sources. Here are the key principles and processes involved:
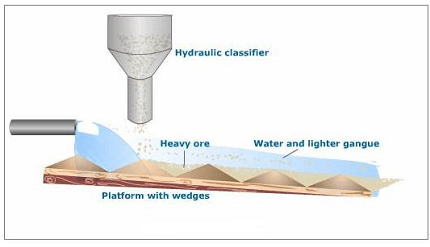
- Concentration of Ores: Most elements are found in nature in the form of minerals or ores, which are often impure and contain other unwanted substances. The first step in isolation is to concentrate the ore by methods like gravity separation, magnetic separation, froth floatation, or leaching. These techniques exploit differences in physical or chemical properties to separate the desired element from the impurities.
- Reduction: Many elements are obtained by reducing their compounds, typically metal oxides or sulfides. Reduction involves the removal of oxygen or another element from the compound, leading to the isolation of the desired element. Reduction can be achieved through various methods, including heating with a reducing agent, electrolysis, or by using chemical reactions.
- Roasting and Calcination: Roasting is the process of heating ores in the presence of excess oxygen, which leads to the conversion of sulfides or carbonates to their respective oxides. Calcination involves heating ores in the absence of oxygen, resulting in the decomposition of carbonates or hydrated compounds. Both processes are used to prepare ores for further processing and facilitate the extraction of metals.
- Smelting: Smelting involves the extraction of metals from their ores by heating the concentrated ore with a reducing agent such as coke (carbon) or a suitable metal. This process helps in the reduction of metal oxides, producing molten metal and slag. The molten metal is then separated from the slag, which contains impurities.
- Electrolytic Refining: Some metals, such as copper and aluminum, are obtained through electrolytic refining. In this process, impure metal is used as anode, and a pure metal sheet is used as a cathode. The metal ions migrate from the anode to the cathode under the influence of an electric current, resulting in the deposition of pure metal at the cathode.
- Fractional Distillation: Fractional distillation is a process used to separate mixtures of liquids with different boiling points. This technique is employed in the isolation of certain elements or compounds that are volatile and can be vaporized and condensed at different temperatures.
- Solvent Extraction: Solvent extraction, also known as liquid-liquid extraction, is a technique used to separate compounds based on their solubility in different solvents. This method is commonly used in the isolation of certain elements or compounds from a mixture or solution.
- Chromatography: Chromatography is a separation technique that is based on the differential partitioning of compounds between a stationary phase and a mobile phase. Different types of chromatography, such as paper chromatography or column chromatography, can be employed to separate and isolate elements or compounds.
These are some of the general principles and processes involved in the isolation of elements. The specific methods used depend on the nature of the element, its compound, and the desired purity level required.
The syllabus for the subject of Chemistry in the integrated course AIIMS (All India Institute of Medical Sciences) typically covers various topics, including the general principles and processes of isolation of elements. Here is an overview of the chemistry syllabus related to this topic:
- Occurrence of Elements: Brief introduction to the occurrence of elements in nature, including minerals, ores, and their distribution.
- Minerals and Ores: Classification of minerals and ores, their mode of occurrence, and their chemical composition.
- Extraction of Metals: Principles and methods used in the extraction of metals from their ores, including concentration, roasting, calcination, reduction, and electrolytic reduction.
- Thermodynamics of Metallurgical Processes: Understanding the thermodynamics and energetics involved in the extraction of metals, including concepts like Gibbs free energy, enthalpy, entropy, and spontaneity.
- Electrochemical Principles: Electrochemical methods employed in the extraction of metals, including electrolysis and electrorefining.
- Refining of Metals: Different methods of refining metals to obtain high purity, such as distillation, liquation, zone refining, and electrolytic refining.
- Uses of Metals: Brief overview of the uses and applications of various metals in different fields, including healthcare, industry, and technology.
- Non-Metals: Introduction to the properties, occurrence, and extraction of non-metals, such as carbon, nitrogen, phosphorus, sulfur, and halogens.
- Compounds of Metals: Study of the properties and applications of important compounds of metals, including oxides, halides, sulfides, and carbonates.
- Environmental Chemistry: Understanding the environmental impact of metallurgical processes, including pollution, waste management, and sustainable practices.
It is important to note that the exact syllabus and depth of coverage may vary depending on the specific curriculum of the integrated course AIIMS and any updates made to the syllabus. Therefore, it is advisable to refer to the official syllabus provided by the institution or consult the relevant course materials for the most accurate and up-to-date information.
What is Required AIIMS-SYLLABUS Chemistry syllabus General principles and processes of isolations of elements
Here is a general overview of the chemistry syllabus for AIIMS related to the general principles and processes of isolating elements:
- Introduction to Elements and Compounds: Classification of matter into elements, compounds, and mixtures. Understanding the concept of an element, its symbol, atomic number, and atomic mass.
- Occurrence of Elements: Brief introduction to the occurrence of elements in nature, including minerals, ores, and their distribution.
- Minerals and Ores: Classification of minerals and ores, their mode of occurrence, and their chemical composition.
- Extraction of Metals: Principles and methods used in the extraction of metals from their ores, including concentration, roasting, calcination, reduction, and electrolytic reduction.
- Thermodynamics of Metallurgical Processes: Understanding the thermodynamics and energetics involved in the extraction of metals, including concepts like Gibbs free energy, enthalpy, entropy, and spontaneity.
- Electrochemical Principles: Electrochemical methods employed in the extraction of metals, including electrolysis and electrorefining.
- Refining of Metals: Different methods of refining metals to obtain high purity, such as distillation, liquation, zone refining, and electrolytic refining.
- Uses of Metals: Brief overview of the uses and applications of various metals in different fields, including healthcare, industry, and technology.
- Non-Metals: Introduction to the properties, occurrence, and extraction of non-metals, such as carbon, nitrogen, phosphorus, sulfur, and halogens.
- Compounds of Metals: Study of the properties and applications of important compounds of metals, including oxides, halides, sulfides, and carbonates.
- Environmental Chemistry: Understanding the environmental impact of metallurgical processes, including pollution, waste management, and sustainable practices.
Please note that this is a general outline, and the actual syllabus for AIIMS may vary. It is recommended to refer to the official AIIMS syllabus or consult the relevant course materials for the specific topics and depth of coverage.
Case Study on AIIMS-SYLLABUS Chemistry syllabus General principles and processes of isolations of elements
Isolation of Copper from Its Ore
Introduction: In this case study, we will explore the general principles and processes of isolating the element copper from its ore. Copper is a widely used metal with applications in various industries, including electrical wiring, electronics, construction, and more. The isolation of copper involves several steps, including concentration, roasting, smelting, and electrorefining.
Case Study Details:
- Concentration of Ore: The first step in isolating copper from its ore is the concentration of the ore. Copper ores are typically found as sulfide minerals, such as chalcopyrite (CuFeS2). The ore is crushed and ground to a fine powder. Then, it is subjected to various techniques like froth floatation, which exploits the differences in the physical and chemical properties of copper sulfide ore and the gangue (unwanted materials). This process separates the copper ore from the gangue, resulting in a concentrated copper sulfide ore.
- Roasting: After concentration, the concentrated copper ore undergoes roasting. Roasting is carried out in a furnace at high temperatures in the presence of excess oxygen. The purpose of roasting is to convert the copper sulfide ore (CuFeS2) to copper oxide (CuO) and sulfur dioxide (SO2). The reaction can be represented as follows: 2CuFeS2 + 3O2 → 2CuO + 2FeO + 2SO2
- Smelting: The next step is smelting, where the copper oxide obtained from roasting is further processed to obtain metallic copper. The roasted ore is mixed with a suitable reducing agent, such as coke (carbon), and heated in a furnace. The carbon reduces the copper oxide to copper metal, while the impurities react with the flux (a substance that combines with impurities to form slag) and are removed as slag. The overall reaction can be represented as follows: CuO + C → Cu + CO
- Electrorefining: The copper obtained from smelting is not yet pure and may contain impurities like iron, zinc, and sulfur. Electrorefining is employed to obtain high-purity copper. In this process, impure copper is used as the anode, and a thin sheet of pure copper acts as the cathode. Both anode and cathode are immersed in an electrolyte solution containing copper sulfate. When an electric current is passed through the electrolytic cell, copper ions from the impure copper anode migrate to the cathode and get deposited, resulting in the purification of copper. The impurities settle down as anode mud.
Conclusion: Through the case study on the isolation of copper from its ore, we have examined the general principles and processes involved in the extraction and purification of copper. The process includes the concentration of ore, roasting to convert copper sulfide to copper oxide, smelting to obtain metallic copper, and electrorefining to achieve high-purity copper. These principles and processes can be applied to the extraction and isolation of other elements as well, showcasing the general approach used in the isolation of elements from their respective ores.
White paper on AIIMS-SYLLABUS Chemistry syllabus General principles and processes of isolations of elements
Title: General Principles and Processes of Isolation of Elements: A Comprehensive Overview
Abstract:
This white paper provides a comprehensive overview of the general principles and processes involved in the isolation of elements from their respective sources. The isolation of elements plays a crucial role in various industries, including metallurgy, materials science, and chemistry. Understanding these principles and processes is fundamental in harnessing the properties and applications of elements in their pure forms. This paper explores the key concepts and techniques used in the isolation of elements, including concentration, reduction, electrolysis, and refining.
Introduction
The significance of isolating elements and their impact on industrial applications and scientific research are discussed. The importance of understanding the general principles and processes in isolation techniques is emphasized.
Occurrence of Elements
An overview of the occurrence of elements in nature, including minerals, ores, and their distribution, is provided. The classification of minerals and ores based on their composition and mode of occurrence is discussed.
Concentration of Ores
The process of concentration of ores, involving various techniques such as gravity separation, magnetic separation, froth flotation, and leaching, is explained. The objective is to remove impurities and increase the concentration of the desired element.
Reduction
The principles and methods of reduction, including the use of chemical reactions, heating with a reducing agent, and electrolysis, are explored. The reduction process aims to extract metals from their compounds, such as oxides or sulfides, by removing oxygen or another element.
Electrolysis
The principles and applications of electrolysis in the isolation of elements are discussed. The process involves the use of an electric current to drive a non-spontaneous redox reaction, resulting in the deposition of the desired element at the electrode.
Refining of Metals
Different methods of refining metals to obtain high purity are examined. Techniques such as distillation, liquation, zone refining, and electrolytic refining are discussed, highlighting their respective advantages and applications.
Other Isolation Techniques
Additional techniques used in the isolation of elements are explored, including fractional distillation, solvent extraction, and chromatography. These techniques find applications in separating elements or compounds based on their physical or chemical properties.
Environmental Considerations
The environmental impact of the isolation processes and the importance of sustainable practices are addressed. Strategies for reducing pollution, waste management, and the adoption of greener techniques are discussed.
Case Studies
Real-world case studies illustrating the application of general principles and processes in the isolation of specific elements are presented. These case studies demonstrate the practical implementation of various techniques discussed in the paper.
Conclusion
A summary of the key points covered in the paper is provided, emphasizing the importance of understanding the general principles and processes of isolating elements. The potential for further advancements in isolation techniques and their impact on various industries is highlighted.
References
A list of relevant references and sources is provided for further exploration of the topic.
This white paper aims to serve as a comprehensive guide to the general principles and processes involved in the isolation of elements. It provides a valuable resource for researchers, students, and professionals in the fields of chemistry, materials science, and metallurgy. By understanding these principles and processes, scientists and engineers can unlock the full potential of elements in various applications and contribute to the advancement of scientific knowledge.
