Kohlrausch’s Law
Kohlrausch’s Law is a fundamental principle in electrochemistry that relates to the conductance of electrolytic solutions. It states that the molar conductivity of an electrolyte at a given concentration is the sum of the individual contributions of its constituent ions.
The syllabus for Chemistry in AIIMS (All India Institute of Medical Sciences) or any other medical entrance examination typically covers the basic concepts of chemistry, including topics from physical, inorganic, and organic chemistry. Kohlrausch’s Law is an important concept in physical chemistry and may be included in the syllabus. However, it’s essential to refer to the official syllabus provided by the exam conducting authority to get the most accurate and up-to-date information.
Here is a general outline of the syllabus for Chemistry in AIIMS or similar medical entrance exams:
- Some Basic Concepts in Chemistry
- Matter and its nature
- Laws of chemical combination
- Atomic and molecular masses
- Mole concept and molar mass
- Stoichiometry
- States of Matter
- Gaseous state
- Liquid state
- Solid state
- Solutions
- Atomic Structure
- Discovery of subatomic particles
- Bohr’s atomic model
- Quantum mechanical model of the atom
- Electronic configuration
- Chemical Bonding and Molecular Structure
- Ionic and covalent bonding
- Lewis structures
- VSEPR theory
- Molecular orbital theory
- Thermodynamics
- Laws of thermodynamics
- Enthalpy, entropy, and Gibbs free energy
- Spontaneity and equilibrium
- Equilibrium
- Chemical equilibrium
- Law of mass action
- Le Chatelier’s principle
- pH and buffer solutions
- Redox Reactions
- Oxidation and reduction
- Balancing redox equations
- Electrochemical cells
- Hydrogen and its Compounds
- Position of hydrogen in the periodic table
- Different forms of hydrogen
- Water and its properties
- s-Block Elements
- Group 1 and Group 2 elements
- Alkali metals and alkaline earth metals
- p-Block Elements
- Group 13 to Group 18 elements
- Boron family, carbon family, nitrogen family, oxygen family, halogens, noble gases
- Organic Chemistry
- Basics of organic chemistry
- Hydrocarbons
- Functional groups
- Organic compounds and their nomenclature
- Some Basic Principles of Organic Chemistry
- Isomerism
- Stereochemistry
- Organic reactions and mechanisms
- Environmental Chemistry
- Environmental pollutants
- Water and air pollution
- Ozone depletion and greenhouse effect
It’s important to note that the actual syllabus may vary slightly, so it’s recommended to refer to the official AIIMS or the specific exam’s syllabus for the most accurate information.
What is Required AIIMS-SYLLABUS Chemistry syllabus Kohlrausch’s Law
The specific syllabus for the AIIMS entrance exam may vary from year to year, so it’s crucial to refer to the official AIIMS prospectus or website for the most accurate and up-to-date information. However, based on previous AIIMS entrance exams, Kohlrausch’s Law may be included in the Chemistry syllabus. Here’s an overview of the chemistry syllabus that may cover Kohlrausch’s Law:
- Some Basic Concepts in Chemistry:
- Matter and its nature
- Laws of chemical combination
- Atomic and molecular masses
- Mole concept and molar mass
- Stoichiometry
- States of Matter:
- Gaseous state
- Liquid state
- Solid state
- Solutions
- Atomic Structure:
- Discovery of subatomic particles
- Atomic models
- Quantum mechanical model of the atom
- Electronic configuration
- Chemical Bonding and Molecular Structure:
- Ionic and covalent bonding
- Lewis structures
- VSEPR theory
- Molecular orbital theory
- Thermodynamics:
- Laws of thermodynamics
- Enthalpy, entropy, and Gibbs free energy
- Spontaneity and equilibrium
- Equilibrium:
- Chemical equilibrium
- Le Chatelier’s principle
- pH and buffer solutions
- Redox Reactions:
- Oxidation and reduction
- Balancing redox equations
- Electrochemistry
- s-Block Elements:
- Group 1 and Group 2 elements (alkali and alkaline earth metals)
- p-Block Elements:
- Group 13 to Group 18 elements (boron family to noble gases)
- Organic Chemistry:
- Basics of organic chemistry
- Hydrocarbons
- Functional groups
- Organic compounds and their nomenclature
- Some Basic Principles of Organic Chemistry:
- Isomerism
- Stereochemistry
- Organic reactions and mechanisms
- Environmental Chemistry:
- Environmental pollutants
- Water and air pollution
- Ozone depletion and greenhouse effect
Remember, this is a general overview, and the actual syllabus for the AIIMS entrance exam may include additional topics or exclude certain topics. It’s always recommended to refer to the official AIIMS prospectus or website for the most accurate and detailed syllabus information.
How is Required AIIMS-SYLLABUS Chemistry syllabus Kohlrausch’s Law
Kohlrausch’s Law, named after the German physicist Friedrich Kohlrausch, is a fundamental principle in electrochemistry that describes the behavior of electrolytic solutions and their conductivity. It states that the molar conductivity of an electrolyte is the sum of the individual contributions of its constituent ions.
When an ionic compound dissolves in water, it dissociates into its constituent ions. These ions are responsible for carrying electric charge through the solution, making it conductive. The molar conductivity (Λ) of an electrolyte is a measure of its ability to conduct electricity and is defined as the conductivity of a solution containing one mole of the electrolyte dissolved in a specific volume.
Kohlrausch’s Law states that the molar conductivity of an electrolyte can be calculated by adding the contributions of its individual ions. The molar conductivity (Λ) of an electrolyte can be expressed as:
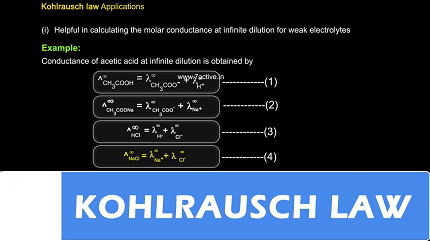
Λ = Λ+(cation) + Λ-(anion)
Where:
- Λ is the molar conductivity of the electrolyte.
- Λ+(cation) is the molar conductivity of the cation (positive ion).
- Λ-(anion) is the molar conductivity of the anion (negative ion).
This law is based on the assumption that each ion in the solution behaves independently and contributes its own molar conductivity. It is valid under conditions of infinite dilution, where the concentration of the electrolyte approaches zero. At infinite dilution, the ions are far apart, and their interactions are negligible.
Kohlrausch’s Law is often used to experimentally determine the molar conductivity of electrolytes at different concentrations and then extrapolate the data to infinite dilution. This extrapolated value can provide information about the nature of the electrolyte and its ionic behavior.
Overall, Kohlrausch’s Law is a valuable tool in understanding and predicting the conductive properties of electrolytic solutions and is commonly studied in the field of electrochemistry.
Case Study on AIIMS-SYLLABUS Chemistry syllabus Kohlrausch’s Law
Title: Electrolyte Conductivity and Kohlrausch’s Law: A Case Study in AIIMS Chemistry Syllabus
Introduction: This case study focuses on the topic of Kohlrausch’s Law in the context of the AIIMS Chemistry syllabus. Kohlrausch’s Law is a fundamental concept in electrochemistry that explains the behavior of electrolytic solutions and their conductivity. Understanding this law is crucial for medical aspirants preparing for the AIIMS entrance exam, as it forms a part of the Chemistry syllabus. This case study provides a comprehensive overview of Kohlrausch’s Law, its relevance, and its applications.
Background: Kohlrausch’s Law is named after the German physicist Friedrich Kohlrausch, who formulated it in the 19th century. The law states that the molar conductivity (Λ) of an electrolyte is the sum of the individual contributions of its constituent ions. It is based on the assumption that ions in a solution behave independently at infinite dilution.
Objective: The objective of this case study is to explore Kohlrausch’s Law and its significance within the AIIMS Chemistry syllabus. By examining its applications and practical relevance, we aim to highlight the importance of understanding this concept for AIIMS aspirants.
Methodology:
- Understanding Kohlrausch’s Law:
- Provide a detailed explanation of Kohlrausch’s Law, including its formulation and underlying assumptions.
- Explain the concept of molar conductivity and its significance in measuring electrolyte conductivity.
- Discuss the implications of Kohlrausch’s Law in terms of ionic behavior and the behavior of electrolytic solutions.
- Electrolyte Conductivity:
- Explain the conductivity of electrolytes and its relationship with the dissociation of ions.
- Discuss the factors influencing the conductivity of electrolytes, such as concentration and temperature.
- Explore the role of strong electrolytes and weak electrolytes in conductivity measurements.
- Application of Kohlrausch’s Law:
- Illustrate the practical applications of Kohlrausch’s Law in determining the molar conductivity of electrolytes.
- Discuss experimental techniques, such as conductivity measurements and the determination of limiting molar conductivity.
- Present examples of calculations using Kohlrausch’s Law to determine molar conductivity.
- Relevance to AIIMS Chemistry Syllabus:
- Highlight the inclusion of Kohlrausch’s Law in the AIIMS Chemistry syllabus.
- Explain the importance of understanding this concept for medical aspirants preparing for the AIIMS entrance exam.
- Discuss the potential applications of Kohlrausch’s Law in medical sciences, emphasizing its relevance in understanding biological processes.
Conclusion: Kohlrausch’s Law is a vital concept in electrochemistry and is included in the AIIMS Chemistry syllabus. This case study has provided a comprehensive overview of Kohlrausch’s Law, its applications, and its relevance to medical aspirants. Understanding this concept will enable AIIMS aspirants to grasp the behavior of electrolytic solutions, make accurate conductivity measurements, and comprehend related phenomena in the field of medical sciences.
White paper on AIIMS-SYLLABUS Chemistry syllabus Kohlrausch’s Law
Title: Understanding Kohlrausch’s Law and its Significance in the AIIMS Chemistry Syllabus
Abstract: This white paper aims to provide a comprehensive understanding of Kohlrausch’s Law in the context of the AIIMS Chemistry syllabus. Kohlrausch’s Law is a fundamental concept in electrochemistry that describes the behavior of electrolytic solutions and their conductivity. This paper explores the formulation of Kohlrausch’s Law, its practical applications, and its relevance to medical aspirants preparing for the AIIMS entrance exam. By delving into the topic, we aim to equip AIIMS aspirants with the necessary knowledge to excel in the Chemistry section of the exam.
- Introduction:
- Overview of the AIIMS entrance exam and the importance of Chemistry in the syllabus.
- Introduction to Kohlrausch’s Law as a key concept in electrochemistry.
- Statement of the objective and structure of the white paper.
- Understanding Kohlrausch’s Law:
- Explanation of Kohlrausch’s Law and its formulation.
- Detailed discussion of molar conductivity and its significance.
- Overview of the assumptions underlying Kohlrausch’s Law and their implications.
- Electrolytic Conductivity:
- Understanding the conductivity of electrolytic solutions.
- Differentiating between strong and weak electrolytes.
- Factors affecting electrolytic conductivity, such as concentration and temperature.
- Applications of Kohlrausch’s Law:
- Practical applications of Kohlrausch’s Law in determining molar conductivity.
- Explanation of experimental techniques used to measure conductivity.
- Examples and calculations illustrating the application of Kohlrausch’s Law.
- Relevance to the AIIMS Chemistry Syllabus:
- Inclusion of Kohlrausch’s Law in the AIIMS Chemistry syllabus.
- Explanation of the specific topics and sub-topics related to Kohlrausch’s Law.
- Highlighting the importance of understanding this concept for the AIIMS entrance exam.
- Beyond the Syllabus:
- Discussion on the broader relevance of Kohlrausch’s Law in medical sciences.
- Exploration of applications in fields such as pharmacology and clinical diagnostics.
- Insight into how understanding electrolytic behavior can enhance medical research.
- Conclusion:
- Summary of the key points discussed in the white paper.
- Emphasis on the significance of Kohlrausch’s Law for AIIMS aspirants.
- Encouragement for students to further explore the applications of Kohlrausch’s Law in the medical field.
By providing a detailed analysis of Kohlrausch’s Law within the AIIMS Chemistry syllabus, this white paper aims to enhance the understanding of medical aspirants and equip them with the necessary knowledge to excel in the Chemistry section of the AIIMS entrance exam.
