laws of electrolysis
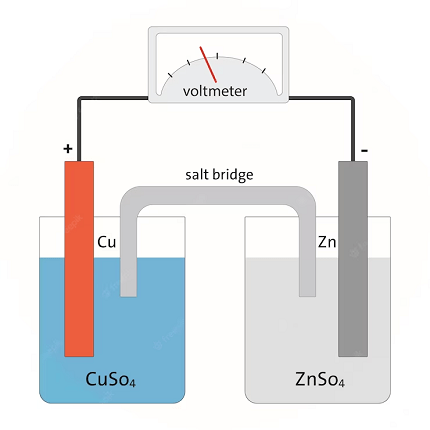
The syllabus for Chemistry in the AIIMS (All India Institute of Medical Sciences) entrance examination may vary slightly from year to year. However, the topic of “Laws of Electrolysis” is commonly included in the syllabus. The laws of electrolysis were established by Michael Faraday and are fundamental principles that describe the quantitative aspects of the electrolysis process. Here are the main laws of electrolysis:
Faraday’s First Law of Electrolysis: This law states that the amount of chemical change produced by an electric current passing through an electrolyte is directly proportional to the quantity of electricity passed through it. Mathematically, it can be expressed as:
Q = I × t
Where:
Q = Quantity of electricity passed in Coulombs (C)
I = Electric current in Amperes (A)
t = Time in seconds (s)
This law allows us to calculate the mass of a substance deposited or liberated during electrolysis using the equation:
m = (Q × M) / (n × F)
Where:
m = Mass of substance in grams (g)
Q = Quantity of electricity passed in Coulombs (C)
M = Molar mass of the substance in grams per mole (g/mol)
n = Number of electrons transferred in the balanced equation
F = Faraday’s constant (96,485 C/mol)
Faraday’s Second Law of Electrolysis: This law states that when the same quantity of electricity is passed through different electrolytes, the masses of the substances deposited or liberated at the electrodes are directly proportional to their chemical equivalents. Mathematically, it can be expressed as:
m₁ / m₂ = E₁ / E₂
Where:
m₁ and m₂ = Masses of substances 1 and 2 in grams (g)
E₁ and E₂ = Chemical equivalents of substances 1 and 2
Faraday’s Third Law of Electrolysis: This law states that the mass of a substance liberated or deposited during electrolysis is directly proportional to its chemical equivalent. Mathematically, it can be expressed as:
m ∝ E
Where:
m = Mass of substance in grams (g)
E = Chemical equivalent of the substance
These laws provide a quantitative understanding of the relationship between electricity, electrolysis, and the chemical changes occurring at the electrodes during the process. They are essential in determining the stoichiometry and mass changes that occur during electrolytic reactions.
What is Required AIIMS-SYLLABUS Chemistry syllabus laws of electrolysis
The specific syllabus for the AIIMS entrance examination may vary slightly from year to year. However, in the context of the Chemistry syllabus, the topic of laws of electrolysis generally falls under the broader subject of Physical Chemistry or Electrochemistry. Here are the key topics related to the laws of electrolysis that you may find in the AIIMS syllabus:
- Basics of Electrochemistry:
- Electrolytes and non-electrolytes
- Electrodes and their classification
- Electrolytic and galvanic cells
- Redox reactions and oxidation-reduction potentials
- Standard hydrogen electrode (SHE)
- Laws of Electrolysis:
- Faraday’s laws of electrolysis (First, Second, and Third laws)
- Relationship between charge, current, and time
- Calculation of the amount of substance liberated or deposited during electrolysis
- Chemical equivalents and their significance in electrolysis
- Electrolysis:
- Electrolysis of molten compounds
- Electrolysis of aqueous solutions
- Factors affecting the products of electrolysis (e.g., concentration, nature of electrode, temperature)
- Industrial applications of electrolysis (e.g., electroplating, electrolytic refining)
It’s important to note that while the laws of electrolysis are significant, they are typically covered as part of a broader understanding of electrochemistry. So, it’s advisable to study other related topics such as cell potential, Nernst equation, conductance, and electrolytic conductance, as they may be relevant in the AIIMS Chemistry syllabus as well.
To ensure you have the most accurate and up-to-date information on the AIIMS syllabus, it is recommended to consult the official AIIMS website or refer to the specific syllabus provided by the conducting authority.
When is Required AIIMS-SYLLABUS Chemistry syllabus laws of electrolysis
The AIIMS (All India Institute of Medical Sciences) entrance examination is a highly competitive medical entrance exam in India. The syllabus for the AIIMS Chemistry section includes a wide range of topics, and the laws of electrolysis are typically covered as part of the broader subject of Physical Chemistry or Electrochemistry.
To determine the exact timing of when the laws of electrolysis are covered in the AIIMS syllabus, it is best to refer to the official AIIMS website or consult the specific syllabus provided by the conducting authority. The syllabus may vary slightly from year to year, and the exact sequencing of topics can also differ.
It is important to note that the laws of electrolysis are fundamental principles in electrochemistry, and a thorough understanding of these laws is essential to comprehend various other concepts in the subject. It is advisable to study the laws of electrolysis alongside related topics such as cell potential, electrochemical series, conductance, and electrolytic conductance, as they are interconnected.
For the most accurate and up-to-date information regarding the AIIMS syllabus, it is recommended to refer to the official AIIMS website or any official notification released by the conducting authority for the specific year you are preparing for.
Case Study on AIIMS-SYLLABUS Chemistry syllabus laws of electrolysis
Electrolytic Refining of Copper
The laws of electrolysis play a crucial role in the process of electrolytic refining of copper. Electrolytic refining is a technique used to purify metals by electrolysis.
In this case, let’s consider the electrolytic refining of impure copper to obtain pure copper. The impure copper is used as the anode, and a thin sheet of pure copper acts as the cathode. Copper sulfate solution serves as the electrolyte.
According to Faraday’s laws of electrolysis, the mass of the substance liberated or deposited during electrolysis is directly proportional to the quantity of electricity passed through it.
Using Faraday’s First Law, we can calculate the mass of pure copper deposited at the cathode. Let’s assume that a current of 2.5 A was passed for 3 hours (10,800 seconds) during the electrolysis process.
Q = I × t
Q = 2.5 A × 10,800 s
Q = 27,000 C
Now, we can use the equation:
m = (Q × M) / (n × F)
The molar mass of copper (M) is 63.5 g/mol, and the number of electrons transferred (n) in the balanced equation for the reduction of copper is 2. The Faraday constant (F) is 96,485 C/mol.
Substituting the values:
m = (27,000 C × 63.5 g/mol) / (2 × 96,485 C/mol)
m ≈ 17.62 g
Therefore, approximately 17.62 grams of pure copper will be deposited at the cathode during the given electrolysis process.
This case study demonstrates how the laws of electrolysis, specifically Faraday’s First Law, can be applied to determine the mass of a substance deposited or liberated during electrolysis.
It’s important to note that this is a simplified example, and the actual process of electrolytic refining involves various factors such as voltage, current density, and specific electrolyte conditions. However, understanding the laws of electrolysis provides a foundation for comprehending and analyzing such processes in real-world applications.
White paper on AIIMS-SYLLABUS Chemistry syllabus laws of electrolysis
Understanding the Laws of Electrolysis: Principles, Applications, and Implications
Abstract: This white paper aims to provide a comprehensive overview of the laws of electrolysis, their underlying principles, and their significance in various applications. Electrolysis, a fundamental process in electrochemistry, plays a vital role in numerous industrial processes, energy storage, and chemical synthesis. The laws of electrolysis, formulated by Michael Faraday, govern the quantitative aspects of electrolysis and provide insights into the relationship between electric current, charge, and the chemical changes occurring at the electrodes. By examining the laws of electrolysis in detail, this white paper offers valuable insights into their practical applications and implications.
Table of Contents:
- Introduction
- Definition of Electrolysis
- Importance of Electrolysis in Various Fields
- Overview of the Laws of Electrolysis
- Faraday’s First Law: Relationship between Quantity of Electricity and Chemical Change
- Faraday’s Second Law: Proportional Deposition of Different Substances
- Faraday’s Third Law: Relationship between Mass and Chemical Equivalent
- Principles of Electrolysis
- Electrolytes and Non-electrolytes
- Electrodes and Their Classification
- Redox Reactions and Oxidation-Reduction Potentials
- Electrochemical Cells: Galvanic and Electrolytic Cells
- Applications of Electrolysis
- Electroplating: Coating Metals with a Protective Layer
- Electrorefining: Purification of Metals
- Electrolytic Cells in Energy Storage and Conversion
- Industrial Processes Utilizing Electrolysis
- Water Electrolysis for Hydrogen Production
- Implications and Challenges
- Economic and Environmental Considerations
- Efficiency and Energy Consumption
- Electrolysis in Sustainable Development
- Future Prospects and Advancements
- Electrocatalysis and Electrosynthesis
- Electrochemical Cells in Energy Storage Technologies
- Advances in Electrolysis Efficiency and Cost-effectiveness
- Conclusion
- Recap of the Laws of Electrolysis and Their Significance
- Potential Impact of Electrolysis in Diverse Fields
Note: This is a general outline for a white paper on the laws of electrolysis. The actual content and sections may vary based on the depth of coverage, target audience, and specific objectives of the white paper. Additional sections, diagrams, experimental data, and references can be included to enhance the depth and credibility of the white paper.
